Smoking cessation strategy in the national cervical cancer screening program (SUCCESS): study protocol for a pragmatic cluster randomised trial and process evaluation in Dutch general practice
- PMID: 35379626
- PMCID: PMC8981275
- DOI: 10.1136/bmjopen-2021-055812
Smoking cessation strategy in the national cervical cancer screening program (SUCCESS): study protocol for a pragmatic cluster randomised trial and process evaluation in Dutch general practice
Abstract
Introduction: Cervical cancer screening in general practice could be a routine moment to provide female smokers with stop smoking advice and support. The aim of this study is to assess the effect of a stop smoking strategy delivered by trained practice assistants after the cervical smear, and to evaluate the implementation process.
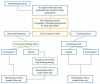
Methods and analysis: The study is a two-arm, pragmatic cluster randomised trial, in Dutch general practice. Randomisation takes place 1:1 at the level of the general practice. Practices either deliver the SUCCESS stop smoking strategy or the usual care condition. The strategy consists of brief stop smoking advice based on the Ask-Advise-Connect method and is conducted by trained practice assistants after routine cervical cancer screening. The primary outcome is the performance of a serious quit attempt in the 6 months after screening. Secondary outcomes are 7-day point prevalence abstinence, reduction in the number of cigarettes per day and transition in motivation to quit smoking. Follow-up for these measurements takes place after 6 months. Analysis on the primary outcome aims to detect a 10% difference between treatment arms (0.80 power, p=0.05, using a one-sided test), and will be performed according to the intention to treat principle. The process evaluation will assess feasibility, acceptability and barriers or enablers to the strategy's implementation. For this purpose, both qualitative and quantitative data will be collected via questionnaires and in-depth interviews, respectively, in both individual study participants and involved staff.
Ethics and dissemination: The Dutch Ministry of Health, Welfare and Sport approved of the trial after an advisory report from the Health Council (Nr. 2018/17). A licence was provided to conduct the study under the Population Screening Act. Study results will be disseminated through publications in peer-reviewed journals and conference presentations.
Trial registration number: NL5052 (NTR7451).
Keywords: preventive medicine; primary care; protocols & guidelines; substance misuse.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures
Similar articles
-
A Stop-Smoking Strategy After Cervical Cancer Screening: Results of a Cluster-Randomized Controlled Trial in Dutch General Practice.Nicotine Tob Res. 2025 Apr 22;27(5):805-814. doi: 10.1093/ntr/ntae285. Nicotine Tob Res. 2025. PMID: 39673389 Clinical Trial.
-
Stop smoking advice by practice assistants after routine cervical screening in general practice: A qualitative exploration of potential barriers and enablers.Eur J Gen Pract. 2022 Dec;28(1):56-65. doi: 10.1080/13814788.2022.2053105. Eur J Gen Pract. 2022. PMID: 35394361 Free PMC article.
-
Effectiveness of training general practitioners to improve the implementation of brief stop-smoking advice in German primary care: study protocol of a pragmatic, 2-arm cluster randomised controlled trial (the ABCII trial).BMC Fam Pract. 2019 Jul 27;20(1):107. doi: 10.1186/s12875-019-0986-8. BMC Fam Pract. 2019. PMID: 31351460 Free PMC article.
-
Effectiveness of nicotine replacement therapy sample at outdoor smoking hotspots for initiating quit attempts and use of smoking cessation services: a protocol for a cluster randomised controlled trial.BMJ Open. 2020 Apr 7;10(4):e036339. doi: 10.1136/bmjopen-2019-036339. BMJ Open. 2020. PMID: 32269028 Free PMC article.
-
The delivery of Ask-Advise-Connect for smoking cessation in Dutch general practice during the COVID-19 pandemic: results of a pre-post implementation study.BMC Health Serv Res. 2023 Jun 19;23(1):654. doi: 10.1186/s12913-023-09692-1. BMC Health Serv Res. 2023. PMID: 37337250 Free PMC article.
References
-
- Lanting C, de Vroome E, Elias S. De bijdrage van leefstijlfactoren AAN de incidentie van en de sterfte AAN kanker in Nederland: TNO; TNO-rapport, 2014. Available: https://www.kwf.nl/sites/default/files/2019-09/eindrapport-paf-studie.pdf
