Diagnostic and prognostic factors in patients with prostate cancer: a systematic review
- PMID: 35379637
- PMCID: PMC8981333
- DOI: 10.1136/bmjopen-2021-058267
Diagnostic and prognostic factors in patients with prostate cancer: a systematic review
Abstract
Objectives: As part of the PIONEER Consortium objectives, we have explored which diagnostic and prognostic factors (DPFs) are available in relation to our previously defined clinician and patient-reported outcomes for prostate cancer (PCa).
Design: We performed a systematic review to identify validated and non-validated studies.
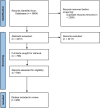
Data sources: MEDLINE, Embase and the Cochrane Library were searched on 21 January 2020.
Eligibility criteria: Only quantitative studies were included. Single studies with fewer than 50 participants, published before 2014 and looking at outcomes which are not prioritised in the PIONEER core outcome set were excluded.
Data extraction and synthesis: After initial screening, we extracted data following the Checklist for Critical Appraisal and Data Extraction for Systematic Reviews of prognostic factor studies (CHARMS-PF) criteria and discussed the identified factors with a multidisciplinary expert group. The quality of the included papers was scored for applicability and risk of bias using validated tools such as PROBAST, Quality in Prognostic Studies and Quality Assessment of Diagnostic Accuracy Studies 2.
Results: The search identified 6604 studies, from which 489 DPFs were included. Sixty-four of those were internally or externally validated. However, only three studies on diagnostic and seven studies on prognostic factors had a low risk of bias and a low risk concerning applicability.
Conclusion: Most of the DPFs identified require additional evaluation and validation in properly designed studies before they can be recommended for use in clinical practice. The PIONEER online search tool for DPFs for PCa will enable researchers to understand the quality of the current research and help them design future studies.
Ethics and dissemination: There are no ethical implications.
Keywords: epidemiology; prostate disease; urological tumours.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures

References
-
- Mottet NBJ, Briers E, Bolla M. Members of the EAU – ESTRO – ESUR –SIOG prostate cancer guidelines panel. EAU – ESTRO – ESUR – SIOG guidelines on prostate cancer. EAU annual Congress. Milan: EAU Guidelines Office, 2021.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
