Human Endothelial Colony-Forming Cells
- PMID: 35379656
- PMCID: PMC9530069
- DOI: 10.1101/cshperspect.a041154
Human Endothelial Colony-Forming Cells
Abstract
Endothelial colony-forming cells (ECFCs) are progenitor cells that can give rise to colonies of highly proliferative vascular endothelial cells (ECs) with clonal expansion and in vivo blood vessel-forming potential. More than two decades ago, the identification of ECFCs in human peripheral blood created tremendous opportunities as having a clinically accessible source of autologous ECs could facilitate meaningful therapies with the potential to impact multiple vascular diseases. Nevertheless, until recently, the field of endothelial progenitor cells has been plagued with ambiguities and controversies, and reaching a consensus on the definition of ECFCs has not been straightforward. Moreover, although the basic phenotypical and functional characteristics of cultured ECFCs are now well established, some fundamental questions such as the origin of ECFCs and their physiological roles in health and disease remain incompletely understood. Here, I highlight some critical studies that have shaped our current understanding of ECFCs in humans. Insights into the biological attributes of ECFCs are essential for facilitating the clinical translation of their therapeutic potential.
Copyright © 2022 Cold Spring Harbor Laboratory Press; all rights reserved.
Figures
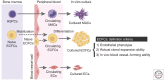
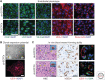
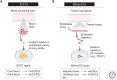
References
-
- Alphonse RS, Vadivel A, Fung M, Shelley WC, Critser PJ, Ionescu L, O'Reilly M, Ohls RK, McConaghy S, Eaton F, et al. 2014. Existence, functional impairment, and lung repair potential of endothelial colony-forming cells in oxygen-induced arrested alveolar growth. Circulation 129: 2144–2157. 10.1161/CIRCULATIONAHA.114.009124 - DOI - PMC - PubMed
-
- Alvarado-Moreno JA, Hernandez-Lopez R, Chavez-Gonzalez A, Yoder MC, Rangel-Corona R, Isordia-Salas I, Hernandez-Juarez J, Cerbulo-Vazquez A, Gonzalez-Jimenez MA, Majluf-Cruz A. 2016. Endothelial colony-forming cells: biological and functional abnormalities in patients with recurrent, unprovoked venous thromboembolic disease. Thromb Res 137: 157–168. 10.1016/j.thromres.2015.11.005 - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
