Metabotropic glutamate receptor 5 knockout rescues obesity phenotype in a mouse model of Huntington's disease
- PMID: 35379852
- PMCID: PMC8980063
- DOI: 10.1038/s41598-022-08924-4
Metabotropic glutamate receptor 5 knockout rescues obesity phenotype in a mouse model of Huntington's disease
Abstract
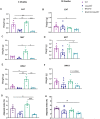
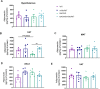
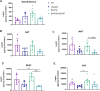
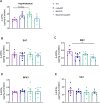
Obesity represents a global health problem and is characterized by metabolic dysfunctions and a low-grade chronic inflammatory state, which can increase the risk of comorbidities, such as atherosclerosis, diabetes and insulin resistance. Here we tested the hypothesis that the genetic deletion of metabotropic glutamate receptor 5 (mGluR5) may rescue metabolic and inflammatory features present in BACHD mice, a mouse model of Huntington's disease (HD) with an obese phenotype. For that, we crossed BACHD and mGluR5 knockout mice (mGluR5-/-) in order to obtain the following groups: Wild type (WT), mGluR5-/-, BACHD and BACHD/mGluR5-/- (double mutant mice). Our results showed that the double mutant mice present decreased body weight as compared to BACHD mice in all tested ages and reduced visceral adiposity as compared to BACHD at 6 months of age. Additionally, 12-month-old double mutant mice present increased adipose tissue levels of adiponectin, decreased leptin levels, and increased IL-10/TNF ratio as compared to BACHD mice. Taken together, our preliminary data propose that the absence of mGluR5 reduce weight gain and visceral adiposity in BACHD mice, along with a decrease in the inflammatory state in the visceral adipose tissue (VAT), which may indicate that mGluR5 may play a role in adiposity modulation.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





Similar articles
-
Metabotropic glutamate receptor 5 ablation accelerates age-related neurodegeneration and neuroinflammation.Neurochem Int. 2019 Jun;126:218-228. doi: 10.1016/j.neuint.2019.03.020. Epub 2019 Mar 29. Neurochem Int. 2019. PMID: 30930274
-
Metabotropic glutamate receptor 5 positive allosteric modulators are neuroprotective in a mouse model of Huntington's disease.Br J Pharmacol. 2013 Jun;169(4):909-21. doi: 10.1111/bph.12164. Br J Pharmacol. 2013. PMID: 23489026 Free PMC article.
-
mGluR5 regulates REST/NRSF signaling through N-cadherin/β-catenin complex in Huntington's disease.Mol Brain. 2020 Aug 28;13(1):118. doi: 10.1186/s13041-020-00657-7. Mol Brain. 2020. PMID: 32859226 Free PMC article.
-
Metabotropic glutamate receptor 5 as a potential therapeutic target in Huntington's disease.Expert Opin Ther Targets. 2014 Nov;18(11):1293-304. doi: 10.1517/14728222.2014.948419. Epub 2014 Aug 14. Expert Opin Ther Targets. 2014. PMID: 25118797 Review.
-
Visceral adiposity and inflammatory bowel disease.Int J Colorectal Dis. 2021 Nov;36(11):2305-2319. doi: 10.1007/s00384-021-03968-w. Epub 2021 Jun 9. Int J Colorectal Dis. 2021. PMID: 34104989 Review.
Cited by
-
mGluR5 ablation leads to age-related synaptic plasticity impairments and does not improve Huntington's disease phenotype.Sci Rep. 2022 May 28;12(1):8982. doi: 10.1038/s41598-022-13029-z. Sci Rep. 2022. PMID: 35643779 Free PMC article.
-
Associations between GRM7 polymorphisms and obesity in patients selected for sleeve gastrectomy.Metab Brain Dis. 2023 Dec;38(8):2765-2771. doi: 10.1007/s11011-023-01313-4. Epub 2023 Oct 26. Metab Brain Dis. 2023. PMID: 37882887
-
Concomitant pathologies and their impact on Huntington's disease. A brief review of current evidence.J Neural Transm (Vienna). 2025 Jun 21. doi: 10.1007/s00702-025-02957-5. Online ahead of print. J Neural Transm (Vienna). 2025. PMID: 40542842 Review. No abstract available.
-
Inhibition of metabotropic glutamate receptor-5 alleviates hepatic steatosis by enhancing autophagy via activation of the AMPK signaling pathway.World J Gastroenterol. 2025 Feb 21;31(7):98852. doi: 10.3748/wjg.v31.i7.98852. World J Gastroenterol. 2025. PMID: 39991675 Free PMC article.
References
-
- Williams EP, Mesidor M, Winters K, Dubbert PM, Wyatt SB. Overweight and obesity: Prevalence, consequences, and causes of a growing public health problem. Curr. Obes. Rep. 2015;4:363–370. - PubMed
-
- Cinti S, et al. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J. Lipid Res. 2005;46:2347–2355. - PubMed
-
- Vendrell J, et al. Resistin, adiponectin, ghrelin, leptin, and proinflammatory cytokines: Relationships in obesity. Obes. Res. 2004;12:962–971. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

