Current advances in the use of exosomes, liposomes, and bioengineered hybrid nanovesicles in cancer detection and therapy
- PMID: 35379933
- PMCID: PMC9622806
- DOI: 10.1038/s41401-022-00902-w
Current advances in the use of exosomes, liposomes, and bioengineered hybrid nanovesicles in cancer detection and therapy
Abstract
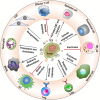
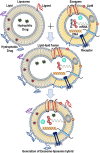
Three major approaches of cancer therapy can be enunciated as delivery of biotherapeutics, tumor image analysis, and immunotherapy. Liposomes, artificial fat bubbles, are long known for their capacity to encapsulate a diverse range of bioactive molecules and release the payload in a sustained, stimuli-responsive manner. They have already been widely explored as a delivery vehicle for therapeutic drugs as well as imaging agents. They are also extensively being used in cancer immunotherapy. On the other hand, exosomes are naturally occurring nanosized extracellular vesicles that serve an important role in cell-cell communication. Importantly, the exosomes also have proven their capability to carry an array of active pharmaceuticals and diagnostic molecules to the tumor cells. Exosomes, being enriched with tumor antigens, have numerous immunomodulatory effects. Much to our intrigue, in recent times, efforts have been directed toward developing smart, bioengineered, exosome-liposome hybrid nanovesicles, which are augmented by the benefits of both vesicular systems. This review attempts to summarize the contemporary developments in the use of exosome and liposome toward cancer diagnosis, therapy, as a vehicle for drug delivery, diagnostic carrier for tumor imaging, and cancer immunotherapy. We shall also briefly reflect upon the recent advancements of the exosome-liposome hybrids in cancer therapy. Finally, we put forward future directions for the use of exosome/liposome and/or hybrid nanocarriers for accurate diagnosis and personalized therapies for cancers.
Keywords: drug delivery; exosome-liposome hybrid; extracellular vesicles (EVs); immunotherapy; liposome; liquid biopsy.
© 2022. The Author(s), under exclusive licence to Shanghai Institute of Materia Medica, Chinese Academy of Sciences and Chinese Pharmacological Society.
Conflict of interest statement
The authors declare no competing interests.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

