Mitotic protein kinase-driven crosstalk of machineries for mitosis and metastasis
- PMID: 35379935
- PMCID: PMC9076678
- DOI: 10.1038/s12276-022-00750-y
Mitotic protein kinase-driven crosstalk of machineries for mitosis and metastasis
Abstract
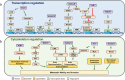
Accumulating evidence indicates that mitotic protein kinases are involved in metastatic migration as well as tumorigenesis. Protein kinases and cytoskeletal proteins play a role in the efficient release of metastatic cells from a tumor mass in the tumor microenvironment, in addition to playing roles in mitosis. Mitotic protein kinases, including Polo-like kinase 1 (PLK1) and Aurora kinases, have been shown to be involved in metastasis in addition to cell proliferation and tumorigenesis, depending on the phosphorylation status and cellular context. Although the genetic programs underlying mitosis and metastasis are different, the same protein kinases and cytoskeletal proteins can participate in both mitosis and cell migration/invasion, resulting in migratory tumors. Cytoskeletal remodeling supports several cellular events, including cell division, movement, and migration. Thus, understanding the contributions of cytoskeletal proteins to the processes of cell division and metastatic motility is crucial for developing efficient therapeutic tools to treat cancer metastases. Here, we identify mitotic kinases that function in cancer metastasis as well as tumorigenesis. Several mitotic kinases, namely, PLK1, Aurora kinases, Rho-associated protein kinase 1, and integrin-linked kinase, are considered in this review, as an understanding of the shared machineries between mitosis and metastasis could be helpful for developing new strategies to treat cancer.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Peng Y, et al. ROCK isoforms differentially modulate cancer cell motility by mechanosensing the substrate stiffness. Acta Biomater. 2019;88:86–101. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

