Intravitreal antisense oligonucleotide sepofarsen in Leber congenital amaurosis type 10: a phase 1b/2 trial
- PMID: 35379979
- PMCID: PMC9117145
- DOI: 10.1038/s41591-022-01755-w
Intravitreal antisense oligonucleotide sepofarsen in Leber congenital amaurosis type 10: a phase 1b/2 trial
Abstract
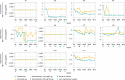
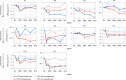
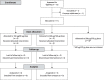
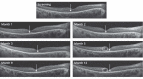
CEP290-associated Leber congenital amaurosis type 10 (LCA10) is a retinal disease resulting in childhood blindness. Sepofarsen is an RNA antisense oligonucleotide targeting the c.2991+1655A>G variant in the CEP290 gene to treat LCA10. In this open-label, phase 1b/2 ( NCT03140969 ), 12-month, multicenter, multiple-dose, dose-escalation trial, six adult patients and five pediatric patients received ≤4 doses of intravitreal sepofarsen into the worse-seeing eye. The primary objective was to evaluate sepofarsen safety and tolerability via the frequency and severity of ocular adverse events (AEs); secondary objectives were to evaluate pharmacokinetics and efficacy via changes in functional outcomes. Six patients received sepofarsen 160 µg/80 µg, and five patients received sepofarsen 320 µg/160 µg. Ten of 11 (90.9%) patients developed ocular AEs in the treated eye (5/6 with 160 µg/80 µg; 5/5 with 320 µg/160 µg) versus one of 11 (9.1%) in the untreated eye; most were mild in severity and dose dependent. Eight patients developed cataracts, of which six (75.0%) were categorized as serious (2/3 with 160 µg/80 µg; 4/5 with 320 µg/160 µg), as lens replacement was required. As the 160-µg/80-µg group showed a better benefit-risk profile, higher doses were discontinued or not initiated. Statistically significant improvements in visual acuity and retinal sensitivity were reported (post hoc analysis). The manageable safety profile and improvements reported in this trial support the continuation of sepofarsen development.
© 2022. The Author(s).
Conflict of interest statement
S.R.R. and A.V.C. report grant funding from ProQR Therapeutics during the conduct of the study. A.V.D. reports grants and other from ProQR Therapeutics during the conduct of the study; personal fees from Medscape and Novartis; grants from Spark Therapeutics and the National Institutes of Health; a patent held by Novartis with royalties paid to Spark Therapeutics and a patent held by the University of Iowa issued to Spark Therapeutics, outside the submitted work. B.P.L. reports grants and non-financial support from ProQR Therapeutics; grants from the Fund for Research Flanders during the conduct of the study; grants from GenSight Therapeutics, IVERIC Bio and Vedere Bio; and grants and non-financial support from Novartis, Spark Therapeutics and RegenXBio, outside the submitted work. C.V.C. reports grants and non-financial support from ProQR Therapeutics, during the conduct of the study, and grants from GenSight Therapeutics, outside the submitted work. A.C.H. reports grants from ProQR Therapeutics both during the conduct of the study and outside the submitted work. E.J. reports grants and non-financial support from ProQR Therapeutics during the conduct of the study and grants from GenSight Therapeutics outside the submitted work. J.D.Z. reports grants from ProQR Therapeutics, during the conduct of the study, and grants from GenSight Therapeutics, outside the submitted work. R.W.J.C. reports other from ProQR Therapeutics, outside the submitted work, and a patent (antisense oligonucleotides) for the treatment of Leber congenital amaurosis licensed to ProQR Therapeutics. P.A. reports share holdings in ProQR Therapeutics. M.E.C. reports grants and personal fees from ProQR Therapeutics, during the conduct of the study, and grants and/or personal fees from Editas Medicine, BridgeBio, PYC and Alia Therapeutics, outside the submitted work. M.R.S., G.P. and A.G. are employees of ProQR Therapeutics. W.D.H., F.A. and D.R. are former employees of ProQR Therapeutics. S.G.J., A.V.D., I.H., M.M., W.P., E.H.S., J.W., A.V.G., A.K.K., C.A.P., A.S., A.J.R., E.V., F.N. and C.H. have nothing to disclose.
Figures




References
Publication types
MeSH terms
Substances
Supplementary concepts
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials

