SIMPA: an open-source toolkit for simulation and image processing for photonics and acoustics
- PMID: 35380031
- PMCID: PMC8978263
- DOI: 10.1117/1.JBO.27.8.083010
SIMPA: an open-source toolkit for simulation and image processing for photonics and acoustics
Abstract
Significance: Optical and acoustic imaging techniques enable noninvasive visualisation of structural and functional properties of tissue. The quantification of measurements, however, remains challenging due to the inverse problems that must be solved. Emerging data-driven approaches are promising, but they rely heavily on the presence of high-quality simulations across a range of wavelengths due to the lack of ground truth knowledge of tissue acoustical and optical properties in realistic settings.
Aim: To facilitate this process, we present the open-source simulation and image processing for photonics and acoustics (SIMPA) Python toolkit. SIMPA is being developed according to modern software design standards.
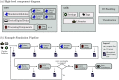
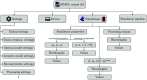
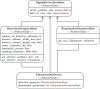
Approach: SIMPA enables the use of computational forward models, data processing algorithms, and digital device twins to simulate realistic images within a single pipeline. SIMPA's module implementations can be seamlessly exchanged as SIMPA abstracts from the concrete implementation of each forward model and builds the simulation pipeline in a modular fashion. Furthermore, SIMPA provides comprehensive libraries of biological structures, such as vessels, as well as optical and acoustic properties and other functionalities for the generation of realistic tissue models.
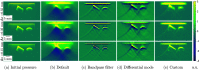
Results: To showcase the capabilities of SIMPA, we show examples in the context of photoacoustic imaging: the diversity of creatable tissue models, the customisability of a simulation pipeline, and the degree of realism of the simulations.
Conclusions: SIMPA is an open-source toolkit that can be used to simulate optical and acoustic imaging modalities. The code is available at: https://github.com/IMSY-DKFZ/simpa, and all of the examples and experiments in this paper can be reproduced using the code available at: https://github.com/IMSY-DKFZ/simpa_paper_experiments.
Keywords: acoustic imaging; open-source; optical imaging; photoacoustics; simulation.
Figures





References
-
- Hauptmann A., Cox B. T., “Deep learning in photoacoustic tomography: current approaches and future directions,” J. Biomed. Opt. 25(11), 112903 (2020). 10.1117/1.JBO.25.11.112903 - DOI

