Long-term efficacy and safety of siponimod in patients with secondary progressive multiple sclerosis: Analysis of EXPAND core and extension data up to >5 years
- PMID: 35380078
- PMCID: PMC9315196
- DOI: 10.1177/13524585221083194
Long-term efficacy and safety of siponimod in patients with secondary progressive multiple sclerosis: Analysis of EXPAND core and extension data up to >5 years
Abstract
Background: Siponimod significantly reduced the risk of confirmed disability progression (CDP), worsening in cognitive processing speed (CPS), relapses, and magnetic resonance imaging (MRI) measures of brain atrophy and inflammation versus placebo in secondary progressive multiple sclerosis (SPMS) patients in the Phase 3 EXPAND study.
Objective: The aim of this study was to assess long-term efficacy and safety of siponimod 2 mg/day from the EXPAND study including the extension part, up to > 5 years.
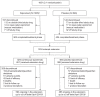
Methods: In the open-label extension part, participants receiving placebo during the core part were switched to siponimod (placebo-siponimod group) and those on siponimod continued the same treatment (continuous siponimod group).
Results: Continuous siponimod reduced the risk of 6-month CDP by 22% (hazard ratio (HR) (95% confidence interval (CI)): 0.78 (0.66-0.92) p = 0.0026) and 6-month confirmed worsening in CPS by 23% (HR (95% CI): 0.77 (0.65-0.92) p = 0.0047) versus the placebo-siponimod group. Sustained efficacy on annualized relapse rate, total and regional brain atrophy, and inflammatory disease activity was also observed. No new, unexpected safety signals for siponimod were identified over the long term.
Conclusion: The sustained efficacy and consistent long-term safety profile of siponimod up to > 5 years support its clinical utility for long-term treatment of SPMS. Benefits in the continuous siponimod versus placebo-siponimod group highlight the significance of earlier treatment initiation.
Trial registration number: NCT01665144.
Keywords: Confirmed disability progression; S1P modulator; confirmed cognitive worsening; cortical gray matter; secondary progressive multiple sclerosis; siponimod.
Conflict of interest statement
Figures











References
-
- Scalfari A, Neuhaus A, Daumer M, et al.. Onset of secondary progressive phase and long-term evolution of multiple sclerosis. J Neurol Neurosurg Psychiatry 2014; 85(1): 67–75. - PubMed
-
- Neuenschwander B, Weber S, Schmidli H, et al.. Predictively consistent prior effective sample sizes. Biometrics 2020; 76: 578–587. - PubMed
-
- Tremlett H, Yinshan Z, Devonshire V. Natural history of secondary-progressive multiple sclerosis. Mult Scler 2008; 14: 314–324. - PubMed
-
- Confavreux C, Vukusic S. Natural history of multiple sclerosis: A unifying concept. Brain 2006; 129(Pt 3): 606–616. - PubMed
-
- National Multiple Sclerosis Society. Types of MS, https://www.nationalmssociety.org/What-is-MS/Types-of-MS (accessed October 2019).

