In vitro and in vivo characterization of Entacapone-loaded nanostructured lipid carriers developed by quality-by-design approach
- PMID: 35380091
- PMCID: PMC8986208
- DOI: 10.1080/10717544.2022.2058651
In vitro and in vivo characterization of Entacapone-loaded nanostructured lipid carriers developed by quality-by-design approach
Abstract
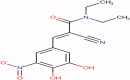
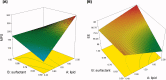
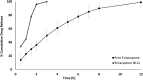
Entacapone, a reversible catechol-o-methyl transferase inhibitor, is used to enhance the action of dopamine agonists by reducing their metabolism and the 'Wearing-off' effects associated with long-term use in the treatment of Parkinson's disease. It is used as an adjunct to levodopa/Carbidopa therapy. Due to limited dissolution and first-pass clearance, it suffers low and variable bioavailability issues. To overcome this problem, the present study aims to explore the potential of nanostructured lipid carriers (NLCs) for the delivery of Entacapone. The Quality by Design (QbD) approach was used for the systematic development of NLCs. The 23 full factorial designs were investigated using Design-Expert®11 software. The three independent variables namely content of total lipid (X1), surfactant (X2), and sonication time (X3) were optimized against two responses namely particle size and entrapment efficiency. The optimized NLCs were characterized for their size, surface morphology, entrapment efficiency, drug release, thermal and crystallographic studies. In-vivo pharmacokinetic studies in Entacapone-loaded NLCs showed an increase in t1/2, AUC0-∞, MRT compared to free drug. The reduction in elimination (Kel) depicts the prolonged action of Entacapone by loading in NLCs. The results displayed Entacapone-loaded NLCs have promising potential for oral delivery and enhanced therapeutic effect which otherwise was a major issue.
Keywords: Entacapone; Parkinson's disease; nanostructured lipid carriers; quality by design.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures






References
-
- Ajiboye AL, Nandi U, Galli M, et al. (2021). Olanzapine loaded nanostructured lipid carriers via high shear homogenization and ultrasonication. Sci Pharm 89:25.
-
- Antonini A, Moro E, Godeiro C, et al. (2018). Medical and surgical management of advanced Parkinson’s disease. Mov Disord 33:900–8. - PubMed
-
- Ashkar A, Sosnik A, Davidovich-Pinhas M. (2021). Structured edible lipid-based particle systems for oral drug-delivery. Biotechnol Adv 54: 107789. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
