Comprehensive profiling of myxopapillary ependymomas identifies a distinct molecular subtype with relapsing disease
- PMID: 35380708
- PMCID: PMC9527524
- DOI: 10.1093/neuonc/noac088
Comprehensive profiling of myxopapillary ependymomas identifies a distinct molecular subtype with relapsing disease
Abstract
Background: Myxopapillary ependymoma (MPE) is a heterogeneous disease regarding histopathology and outcome. The underlying molecular biology is poorly understood, and markers that reliably predict the patients' clinical course are unknown.
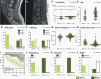
Methods: We assembled a cohort of 185 tumors classified as MPE based on DNA methylation. Methylation patterns, copy number profiles, and MGMT promoter methylation were analyzed for all tumors, 106 tumors were evaluated histomorphologically, and RNA sequencing was performed for 37 cases. Based on methylation profiling, we defined two subtypes MPE-A and MPE-B, and explored associations with epidemiological, clinical, pathological, and molecular characteristics of these tumors.
Results: MPE-A occurred at a median age of 27 years and were enriched with tumors demonstrating papillary morphology and MGMT promoter hypermethylation. Half of these tumors could not be totally resected, and 85% relapsed within 10 years. Copy number alterations were more common in MPE-A. RNA sequencing revealed an enrichment for extracellular matrix and immune system-related signatures in MPE-A. MPE-B occurred at a median age of 45 years and included many tumors with a histological diagnosis of WHO grade II and tanycytic morphology. Patients within this subtype had a significantly better outcome with a relapse rate of 33% in 10 years (P = 3.4e-06).
Conclusions: We unraveled the morphological and clinical heterogeneity of MPE by identifying two molecularly distinct subtypes. These subtypes significantly differed in progression-free survival and will likely need different protocols for surveillance and treatment.
Keywords: DNA methylation; RNA sequencing; histology; myxopapillary ependymoma; outcome.
© The Author(s) 2022. Published by Oxford University Press on behalf of the Society for Neuro-Oncology. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures



References
-
- Bates JE, Choi G, Milano MT. Myxopapillary ependymoma: a SEER analysis of epidemiology and outcomes. J Neurooncol. 2016;129(2):251–258. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

