Cetuximab and Irinotecan With or Without Bevacizumab in Refractory Metastatic Colorectal Cancer: BOND-3, an ACCRU Network Randomized Clinical Trial
- PMID: 35380713
- PMCID: PMC8982431
- DOI: 10.1093/oncolo/oyab025
Cetuximab and Irinotecan With or Without Bevacizumab in Refractory Metastatic Colorectal Cancer: BOND-3, an ACCRU Network Randomized Clinical Trial
Abstract
Background: Combination irinotecan and cetuximab is approved for irinotecan-refractory metastatic colorectal cancer (mCRC). It is unknown if adding bevacizumab improves outcomes.
Patients and methods: In this multicenter, randomized, double-blind, placebo-controlled phase II trial, patients with irinotecan-refractory RAS-wildtype mCRC and no prior anti-EGFR therapy were randomized to cetuximab 500 mg/m2, bevacizumab 5 mg/kg, and irinotecan 180 mg/m2 (or previously tolerated dose) (CBI) versus cetuximab, irinotecan, and placebo (CI) every 2 weeks until disease progression or intolerable toxicity. The primary endpoint was progression-free survival (PFS). Secondary endpoints included overall survival (OS), objective response rate (ORR), and adverse events (AEs).
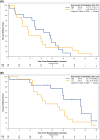
Results: The study closed early after the accrual of 36 out of a planned 120 patients due to changes in funding. Nineteen patients were randomized to CBI and 17 to CI. Baseline characteristics were similar between arms. Median PFS was 9.7 versus 5.5 months for CBI and CI, respectively (1-sided log-rank P = .38; adjusted hazard ratio [HR] = 0.64; 95% confidence interval [CI], 0.25-1.66). Median OS was 19.7 versus 10.2 months for CBI and CI (1-sided log-rank P = .02; adjusted HR = 0.41; 95% CI, 0.15-1.09). ORR was 36.8% for CBI versus 11.8% for CI (P = .13). Grade 3 or higher AEs occurred in 47% of patients receiving CBI versus 35% for CI (P = .46).
Conclusion: In this prematurely discontinued trial, there was no significant difference in the primary endpoint of PFS between CBI and CI. There was a statistically significant improvement in OS in favor of CBI compared with CI. Further investigation of CBI for the treatment of irinotecan-refractory mCRC is warranted.Clinical Trial Registration: NCT02292758.
Keywords: bevacizumab; cetuximab; colorectal neoplasm; irinotecan.
© The Author(s) 2022. Published by Oxford University Press.
Figures
References
-
- Siegel RL, Miller KD, Goding Sauer A, et al. . Colorectal cancer statistics, 2020. CA Cancer J Clin. 2020;70(3):145-164. - PubMed
-
- Holch JW, Ricard I, Stintzing S, Modest DP, Heinemann V.. The relevance of primary tumour location in patients with metastatic colorectal cancer: a meta-analysis of first-line clinical trials. Eur J Cancer. 2017;70:87-98. - PubMed
-
- Brulé SY, Jonker DJ, Karapetis CS, et al. . Location of colon cancer (right-sided versus left-sided) as a prognostic factor and a predictor of benefit from cetuximab in NCIC CO.17. Eur J Cancer. 2015;51(11):1405-1414. - PubMed
-
- Cunningham D, Humblet Y, Siena S, et al. . Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med. 2004;351(4):337-345. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

