Exposure-Response Characterization of Tofacitinib Efficacy in Moderate to Severe Ulcerative Colitis: Results From Phase II and Phase III Induction and Maintenance Studies
- PMID: 35380740
- PMCID: PMC9322343
- DOI: 10.1002/cpt.2601
Exposure-Response Characterization of Tofacitinib Efficacy in Moderate to Severe Ulcerative Colitis: Results From Phase II and Phase III Induction and Maintenance Studies
Abstract
Tofacitinib is an oral small molecule JAK inhibitor for the treatment of ulcerative colitis. Relationships between plasma tofacitinib concentration and efficacy were characterized using exposure-response (E-R) models, with demographic and disease covariates evaluated as potential predictors of efficacy. Data were from phase II and III (OCTAVE Induction 1 and 2) induction studies, and a phase III maintenance study (OCTAVE Sustain). Induction studies included 1,355 patients (tofacitinib 0.5, 3, 10, or 15 mg b.i.d. or placebo). The maintenance study included 592 patients (tofacitinib 5 or 10 mg b.i.d. or placebo). E-R models, including induction patients predicted placebo-adjusted remission rates of 6.4% and 12.7% at week 8 for tofacitinib 5 and 10 mg b.i.d., respectively; corresponding rates in patients without prior tumor necrosis factor inhibitor (TNFi) failure were 12.8% and 20.4%. Estimates to achieve/maintain remission at week 52 of maintenance were 29% and 18% (tofacitinib 5 mg b.i.d.), and 41% and 26% (tofacitinib 10 mg b.i.d.), for patients in remission or not following induction, respectively. During maintenance, patients with prior TNFi failure had lower probability of remission on 5 mg b.i.d. (24.9%) than 10 mg b.i.d. (35.0%). Results indicated tofacitinib 10 mg b.i.d. was an appropriate induction dose but suggested efficacy with 5 mg b.i.d. in patients without prior TNFi failure. Tofacitinib 5 mg b.i.d. was efficacious for maintenance, although patients with prior TNFi failure might see additional benefit on 10 mg b.i.d. Per product labeling, recommended tofacitinib induction dose is 10 mg b.i.d., then maintenance at 5 mg b.i.d. For patients who lose response during maintenance, 10 mg b.i.d. may be considered, limited to the shortest duration. Clinicaltrials.gov: NCT00787202; NCT01465763; NCT01458951; and NCT01458574.
© 2022 Pfizer Inc. Clinical Pharmacology & Therapeutics published by Wiley Periodicals LLC on behalf of American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
A.M., S.T., T.N., J.A.C., I.M., and C.S. are employees and stockholders of Pfizer Inc. G.R.D’H. has served as an advisor for AbbVie, Ablynx, Amakem, AM Pharma, Avaxia, Biogen, Bristol‐Myers Squibb, Boehringer Ingelheim, Celgene, Celltrion, Cosmo, Covidien, Dr. Falk Pharma, Engene, Ferring, Galapagos, Gilead Sciences, GSK, Hospira, Johnson and Johnson, Medimetrics, Millennium/Takeda, Mitsubishi Pharma, MSD, Mundipharma, Novo Nordisk, Pfizer Inc, Prometheus Laboratories/Nestle, Receptos, Robarts Clinical Trials, Salix, Sandoz, Setpoint, Shire, Teva, TiGenix, Tillotts, Topivert, Versant, and Vifor; and has received speaker fees from AbbVie, Ferring, Johnson and Johnson, Millennium/Takeda, MSD, Mundipharma, Norgine, Pfizer Inc, Shire, Tillotts, and Vifor. W.J.S. reports grants, personal fees, and non‐financial support from Pfizer Inc during the conduct of the study; research grants from AbbVie, Abivax, Arena Pharmaceuticals, Boehringer Ingelheim, Celgene, Genentech, Gilead Sciences, GSK, Janssen, Lilly, Pfizer Inc, Prometheus Biosciences, Seres Therapeutics, Shire, Takeda, and Theravance Biopharma; consulting fees from AbbVie, Abivax, AdMIRx, Alfasigma, Alimentiv (previously Robarts Clinical Trials, owned by Alimentiv Health Trust), Alivio Therapeutics, Allakos, Amgen, Applied Molecular Transport, Arena Pharmaceuticals, Bausch Health (Salix), BeiGene, Bellatrix Pharmaceuticals, Boehringer Ingelheim, Boston Pharmaceuticals, Bristol‐Meyers Squibb, Celgene, Celltrion, Cellularity, Cosmo Pharmaceuticals, Escalier Biosciences, Equillium, Forbion, Genentech/Roche, Gilead Sciences, Glenmark Pharmaceuticals, Gossamer Bio, Immunic (Vital Therapies), Index Pharmaceuticals, Intact Therapeutics, Janssen, Kyverna Therapeutics, Landos Biopharma, Lilly, Oppilan Pharma, Otsuka, Pandion Therapeutics, Pfizer Inc, Progenity, Prometheus Biosciences, Prometheus Laboratories, Protagonists Therapeutics, Provention Bio, Reistone Biopharma, Seres Therapeutics, Shanghai Pharma Biotherapeutics, Shire, Shoreline Biosciences, Sublimity Therapeutics, Surrozen, Takeda, Theravance Biopharma, Thetis Pharmaceuticals, Tillotts Pharma, UCB, Vedanta Biosciences, Ventyx Biosciences, Vimalan Biosciences, Vivelix Pharmaceuticals, Vivreon Biosciences, and Zealand Pharma; stock or stock options from Allakos, BeiGene, Gossamer Bio, Oppilan Pharma, Prometheus Biosciences, Prometheus Laboratories Progenity, Shoreline Biosciences, Ventyx Biosciences, Vimalan Biosciences, and Vivreon Biosciences; and employment at Shoreline Biosciences. W.J.S.’s spouse reports consulting fees from Iveric Bio, Oppilan Pharma, and Prometheus Laboratories; stock options from Iveric Bio, Oppilan Pharma, Prometheus Biosciences, Prometheus Laboratories, Ventyx Biosciences, and Vimalan Biosciences; and stock from Progenity, Prometheus Biosciences, Ventyx Biosciences, and Vimalan Biosciences.
Figures
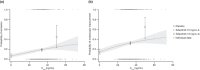


References
-
- Rubin, D.T. , Ananthakrishnan, A.N. , Siegel, C.A. , Sauer, B.G. & Long, M.D. ACG clinical guideline: ulcerative colitis in adults. Am. J. Gastroenterol. 114, 384–413 (2019). - PubMed
-
- Harbord, M. et al. Third European evidence‐based consensus on diagnosis and management of ulcerative colitis. Part 2: current management. J. Crohns Colitis 11, 769–784 (2017). - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

