Recent advances in lipid nanoparticles for delivery of nucleic acid, mRNA, and gene editing-based therapeutics
- PMID: 35381574
- PMCID: PMC9363157
- DOI: 10.1016/j.dmpk.2022.100450
Recent advances in lipid nanoparticles for delivery of nucleic acid, mRNA, and gene editing-based therapeutics
Abstract
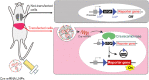
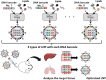
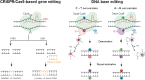
Lipid nanoparticles (LNPs) are becoming popular as a means of delivering therapeutics, including those based on nucleic acids and mRNA. The mRNA-based coronavirus disease 2019 vaccines are perfect examples to highlight the role played by drug delivery systems in advancing human health. The fundamentals of LNPs for the delivery of nucleic acid- and mRNA-based therapeutics, are well established. Thus, future research on LNPs will focus on addressing the following: expanding the scope of drug delivery to different constituents of the human body, expanding the number of diseases that can be targeted, and studying the change in the pharmacokinetics of LNPs under physiological and pathological conditions. This review article provides an overview of recent advances aimed at expanding the application of LNPs, focusing on the pharmacokinetics and advantages of LNPs. In addition, analytical techniques, library construction and screening, rational design, active targeting, and applicability to gene editing therapy have also been discussed.
Keywords: Base editing; DNA barcode; Gene editing; Ionizable lipid; Lipid nanoparticle; Nucleic acid; Positron emission tomography; Recombination; Targeting; mRNA.
Copyright © 2022 The Japanese Society for the Study of Xenobiotics. Published by Elsevier Ltd. All rights reserved.
Figures

References
-
- Kulkarni J.A., Witzigmann D., Chen S., Cullis P.R., Van Der Meel R. Lipid nanoparticle technology for clinical translation of siRNA therapeutics. Acc Chem Res. 2019;52(9):2435–2444. - PubMed
-
- Samaridou E., Heyes J., Lutwyche P. Lipid nanoparticles for nucleic acid delivery: current perspectives. Adv Drug Deliv Rev. 2020;154–155:37–63. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

