[Efficacy of basiliximab in the treatment of 87 cases of steroid-refractory or steroid-dependent acute graft-versus-host disease]
- PMID: 35381672
- PMCID: PMC8980651
- DOI: 10.3760/cma.j.issn.0253-2727.2022.02.006
[Efficacy of basiliximab in the treatment of 87 cases of steroid-refractory or steroid-dependent acute graft-versus-host disease]
Abstract
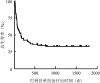
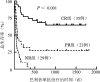
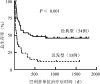
Objective: To evaluate the efficacy and prognosis of basiliximab in the treatment of steroid-refractory or steroid-dependent acute graft-versus-host disease (SR/SD-aGVHD) after allogeneic hematopoietic stem cell transplantation (allo-HSCT) . Methods: Clinical data of 87 patients with SR/SD-aGVHD in the skin, intestine, and liver after allo-HSCT at the Institute of Hematology & Blood Diseases Hospital Transplantation Center from January 2015 to December 2018 were retrospectively analyzed. The administration plan of basiliximab was as follows: 20 mg for adults and children weighing ≥35 kg and 10 mg for children weighing<35 kg. The drug was administered once on the 1st, 4th, and 8th days, respectively, and then once weekly. The efficacy was evaluated on the 7th, 14th, 21st, and 28th days after basiliximab treatment. Results: ①There were 51 males (58.6%) and 36 females (41.4%) , with a median (range) age of 34 (4-63) years. There were 54 cases of classic aGVHD, 33 of late aGVHD, 49 of steroid-refractory aGVHD, and 38 of steroid-dependent aGVHD. ②Thirty-five patients (40.2%) achieved complete remission (CR) , 23 (26.4%) achieved partial remission (PR) , and 29 had no remission (NR) . The total effective rate[overall response rate (ORR) ] was 66.7% (58/87) . ③The ORR of the classic and late aGVHD groups was 77.8% (42/54) and 48.5% (16/33) , respectively. ④The median (range) follow-up time was 154 (4-1813) days, the 6-month overall survival (OS) rate of the 87 patients was 44.8% (95% CI 39.5%-50.1%) and the 1-year OS was 39.4% (95%CI 34.2%-44.3%) . ⑤After treatment with basiliximab, the 6-month OS in the CR (35 cases) , PR (23 cases) , and NR (29 cases) groups was 80.0% (95%CI 73.2%-86.8%) , 39.1% (95%CI 28.9%-49.3%) , and 6.9% (95%CI 2.2%-11.6%) , respectively (χ(2)=34.679, P<0.001) , and the 1-year OS was 74.3% (95%CI 66.9%-81.7%) , 30.4% (95%CI 20.8%-40.0%) , and 3.4% (95%CI 0%-6.8%) , respectively (χ(2)=43.339, P<0.001) . The OS of the classic and late aGVHD groups was 57.4% (95%CI 50.7%-64.1%) and 24.2% (95%CI 16.7%-31.7%) , respectively (χ(2)=9.109, P=0.004) , and the 1-year OS was 51.9% (95%CI 45.1%-58.7%) and 18.2% (95%CI 11.5%-24.9%) , respectively (χ(2)=9.753, P=0.003) . ⑥Univariate and multivariate analyses showed that late aGVHD (OR=3.121, 95%CI 1.770-5.503, P<0.001) , Minnesota score high-risk group before medication (OR=3.591, 95%CI 1.931-6.679, P<0.001) , active infection before medication (OR=1.881, 95%CI 1.029-3.438, P=0.040) , and impairment of important organ function caused by non-GVHD (OR=3.100, 95%CI 1.570-6.121, P=0.001) were independent risk factors affecting the efficacy of basiliximab. Conclusion: Basiliximab has good efficacy and safety for SR/SD-aGVHD, but not in patients with late aGVHD, high-risk group of Minnesota score, and infection or impaired function of important organs.
目的: 分析巴利昔单抗治疗异基因造血干细胞移植后糖皮质激素耐药/依赖急性移植物抗宿主病(aGVHD)患者的疗效。 方法: 对2015年1月至2018年12月期间于中国医学科学院血液病医院行异基因造血干细胞移植后发生皮肤、肠道、肝脏aGVHD并应用巴利昔单抗治疗的87例血液病患者进行回顾性分析。巴利昔单抗用药方案:成人及体重≥35 kg儿童每次20 mg,体重<35 kg儿童每次10 mg,第1、4、8天各给药1次,以后每周用药1次,在巴利昔单抗治疗后第7、14、21、28、42、56天分别进行疗效评估。 结果: ①87例患者中,男51例(58.6%),女36例(41.4%),中位年龄34(4~63)岁,经典型aGVHD 54例,迟发型aGVHD 33例,糖皮质激素耐药49例,糖皮质激素依赖38例。②35例(40.2%)获得完全缓解(CR),23例(26.4%)获得部分缓解(PR),29例患者未缓解(NR),总有效率(ORR)为66.7%(58/87)。③经典型aGVHD组、迟发型aGVHD组ORR分别为77.8%(42/54)、48.5%(16/33)。④中位随访154(4~1813)d,全部87例患者移植后6个月总生存(OS)率为44.8%(95%CI 39.5%~50.1%),移植后1年OS率为39.4%(95%CI 34.2%~44.3%)。⑤巴利昔单抗治疗后CR组(35例)、PR组(23例)、NR组(29例)移植后6个月OS率分别为80.0%(95%CI 73.2%~86.8%)、39.1%(95%CI 28.9%~49.3%)、6.9%(95%CI 2.2%~11.6%)(χ(2)=34.679,P<0.001),移植后1年OS率分别为74.3%(95%CI 66.9%~81.7%)、30.4%(95%CI 20.8%~40.0%)、3.4%(95%CI 0%~6.8%)(χ(2)=43.339,P<0.001)。经典型aGVHD组、迟发型aGVHD组移植后6个月OS率分别为57.4%(95%CI 50.7%~64.1%)、24.2%(95%CI 16.7%~31.7%)(χ(2)=9.109,P=0.004),移植后1年OS率分别为51.9%(95%CI 45.1%~58.7%)、18.2%(95%CI 11.5%~24.9%)(χ(2)=9.753,P=0.003)。⑥单因素及多因素分析结果显示,迟发型aGVHD(OR=3.121,95%CI 1.770~5.503,P<0.001)、用药前明尼苏达积分高危组(OR=3.591,95%CI 1.931~6.679,P<0.001)、用药前存在活动性感染(OR=1.881,95%CI 1.029~3.438,P=0.040)、伴有非GVHD所致重要脏器功能受损(OR=3.100,95%CI 1.570~6.121,P=0.001)是影响巴利昔单抗疗效的独立危险因素。 结论: 巴利昔单抗对于糖皮质激素耐药/依赖aGVHD具有良好的疗效和安全性;迟发型、用药前明尼苏达积分高危组、存在感染或重要脏器功能受损患者疗效不佳。.
Keywords: Basiliximab; Graft-versus-host disease; Hematopoietic stem cell transplantation.
Conflict of interest statement
Figures
Similar articles
-
Basiliximab Treatment for Patients With Steroid-Refractory Acute Graft-Versus-Host Disease Following Matched Sibling Donor Hematopoietic Stem Cell Transplantation.Cell Transplant. 2024 Jan-Dec;33:9636897241257568. doi: 10.1177/09636897241257568. Cell Transplant. 2024. PMID: 38832653 Free PMC article.
-
Vedolizumab plus basiliximab as second-line therapy for steroid-refractory lower gastrointestinal acute graft-versus-host disease.Front Immunol. 2024 Jul 3;15:1408211. doi: 10.3389/fimmu.2024.1408211. eCollection 2024. Front Immunol. 2024. PMID: 39021571 Free PMC article.
-
[Anti-CD(25) monoclonal antibody as a salvage therapy for steroid-refractory acute graft-versus-host disease in 80 patients receiving allogeneic hematopoietic stem cell transplantation].Zhonghua Nei Ke Za Zhi. 2018 May 1;57(5):324-329. doi: 10.3760/cma.j.issn.0578-1426.2018.05.004. Zhonghua Nei Ke Za Zhi. 2018. PMID: 29747286 Chinese.
-
Efficacy of Mesenchymal Stem Cell Therapy for Steroid-Refractory Acute Graft-Versus-Host Disease following Allogeneic Hematopoietic Stem Cell Transplantation: A Systematic Review and Meta-Analysis.PLoS One. 2015 Aug 31;10(8):e0136991. doi: 10.1371/journal.pone.0136991. eCollection 2015. PLoS One. 2015. PMID: 26323092 Free PMC article.
-
Meta-Analysis of Interleukin-2 Receptor Antagonists as the Treatment for Steroid-Refractory Acute Graft-Versus-Host Disease.Front Immunol. 2021 Sep 21;12:749266. doi: 10.3389/fimmu.2021.749266. eCollection 2021. Front Immunol. 2021. PMID: 34621279 Free PMC article.
Cited by
-
[Short-term substitution of calcineurin inhibitors (CNI) with recombinant humanized anti-CD25 monoclonal antibody (Basiliximab) as aGVHD prophylaxis in CNI intolerant patients after allogeneic hematopoietic stem cell transplantation].Zhonghua Xue Ye Xue Za Zhi. 2024 Feb 14;45(2):115-120. doi: 10.3760/cma.j.cn121090-20230519-00201. Zhonghua Xue Ye Xue Za Zhi. 2024. PMID: 38604786 Free PMC article. Chinese.
-
Basiliximab Treatment for Patients With Steroid-Refractory Acute Graft-Versus-Host Disease Following Matched Sibling Donor Hematopoietic Stem Cell Transplantation.Cell Transplant. 2024 Jan-Dec;33:9636897241257568. doi: 10.1177/09636897241257568. Cell Transplant. 2024. PMID: 38832653 Free PMC article.
References
-
- Penack O, Marchetti M, Ruutu T, et al. Prophylaxis and management of graft versus host disease after stem-cell transplantation for haematological malignancies: updated consensus recommendations of the European Society for Blood and Marrow Transplantation[J] Lancet Haematol. 2020;7(2):e157–e167. doi: 10.1016/S2352-3026(19)30256-X. - DOI - PubMed
-
- Wang JZ, Liu KY, Xu LP, et al. Basiliximab for the treatment of steroid-refractory acute graft-versus-host disease after unmanipulated HLA-mismatched/haploidentical hematopoietic stem cell transplantation[J] Transplant Proc. 2011;43(5):1928–1933. doi: 10.1016/j.transproceed.2011.03.044. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials