Overcoming peri- and ortho-selectivity in C-H methylation of 1-naphthaldehydes by a tunable transient ligand strategy
- PMID: 35382469
- PMCID: PMC8906006
- DOI: 10.1039/d1sc05899a
Overcoming peri- and ortho-selectivity in C-H methylation of 1-naphthaldehydes by a tunable transient ligand strategy
Abstract
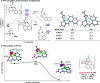
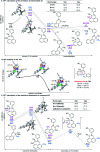
Methyl groups widely exist in bioactive molecules, and site-specific methylation has become a valuable strategy for their structural functionalization. Aiming to introduce this smallest alkyl handle, a highly regioselective peri- and ortho-C-H methylation of 1-naphthaldehyde by using a transient ligand strategy has been developed. A series of methyl-substituted naphthalene frameworks have been prepared in moderate to excellent yields. Mechanistic studies demonstrate that peri-methylation is controlled by the higher electronic density of the peri-position of 1-naphthaldehyde as well as the formation of intermediary 5,6-fused bicyclic palladacycles, whereas experimental studies and theoretical calculations inferred that a 5-membered iridacycle at the ortho-position of 1-naphthaldehyde leads to energetically favorable ortho-methylation via an interconversion between the peri-iridacycle and ortho-iridacycle. Importantly, to demonstrate the synthetic utility of this method, we show that this strategy can serve as a platform for the synthesis of multi-substituted naphthalene-based bioactive molecules and natural products.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures





References
-
- Yan G. Borah A. J. Wang L. Yang M. Adv. Synth. Catal. 2015;357:1333–1350. doi: 10.1002/adsc.201400984. - DOI
-
- Okumura S. Tang S. Saito T. Semba K. Sakaki S. Nakao Y. J. Am. Chem. Soc. 2016;138:14699–14704. doi: 10.1021/jacs.6b08767. - DOI - PubMed
- Liang S. Bolte M. Manolikakes G. Chem.–Eur. J. 2017;23:96–100. doi: 10.1002/chem.201605101. - DOI - PubMed
- Li J. Wang Y. Yu Y. Wu R. Weng J. Lu G. ACS Catal. 2017;7:2661–2667. doi: 10.1021/acscatal.6b03671. - DOI
- Jing C. Chen X. Sun K. Yang Y. Chen T. Liu Y. Qu L. Zhao Y. Yu B. Org. Lett. 2019;21:486–489. doi: 10.1021/acs.orglett.8b03768. - DOI - PubMed
- Zhang M. Luo A. Shi Y. Su R. Yang Y. You J. ACS Catal. 2019;9:11802–11807. doi: 10.1021/acscatal.9b04352. - DOI
- Prévost S. ChemPlusChem. 2020;85:476–486. doi: 10.1002/cplu.202000005. - DOI - PubMed
- Large B. Prim D. Synthesis. 2020;52:2600–2612. doi: 10.1055/s-0040-1707855. - DOI
-
-
Examples of picolinamide directed C8–H founctionalization of naphthalene, see:
- Huang L. Li Q. Wang C. Qi C. J. Org. Chem. 2013;78:3030–3038. doi: 10.1021/jo400017v. - DOI - PubMed
- Nadres E. T. Santos G. I. F. Shabashov D. Daugulis O. J. Org. Chem. 2013;78:9689–9714. doi: 10.1021/jo4013628. - DOI - PMC - PubMed
- Odani R. Hirano K. Satoh T. Miura M. J. Org. Chem. 2013;78:11045–11052. doi: 10.1021/jo402078q. - DOI - PubMed
- Huang L. Sun X. Li Q. Qi C. J. Org. Chem. 2014;79:6720–6725. doi: 10.1021/jo500932x. - DOI - PubMed
- Shang R. Ilies L. Nakamura E. J. Am. Chem. Soc. 2015;137:7660–7663. doi: 10.1021/jacs.5b04818. - DOI - PubMed
- Li Z. Sun S. Qiao H. Yang F. Zhu Y. Kang J. Wu Y. Wu Y. Org. Lett. 2016;18:4594–4597. doi: 10.1021/acs.orglett.6b02243. - DOI - PubMed
- Xiong Y. Yu Y. Weng J. Lu G. Org. Chem. Front. 2018;5:982–989. doi: 10.1039/C7QO01016H. - DOI
- Rej S. Chatani N. ACS Catal. 2018;8:6699–6706. doi: 10.1021/acscatal.8b01675. - DOI
- Zhang T. Zhu H. Yang F. Wu Y. Wu Y. Tetrahedron. 2019;75:1541–1547. doi: 10.1016/j.tet.2019.02.002. - DOI
- Yu X. Yang F. Wu Y. Wu Y. Org. Lett. 2019;21:1726–1729. doi: 10.1021/acs.orglett.9b00283. - DOI - PubMed
- Yao Y. Lin Q. Yang W. Yang W. Gu F. Guo W. Yang D. Chem.–Eur. J. 2020;26:5607–5610. doi: 10.1002/chem.202000411. - DOI - PubMed
- Zu C. Zhang T. Yang F. Wu Y. Wu Y. J. Org. Chem. 2020;85:12777–12784. doi: 10.1021/acs.joc.0c01672. - DOI - PubMed
-
LinkOut - more resources
Full Text Sources

