Recent advances in heterostructured cathodic electrocatalysts for non-aqueous Li-O2 batteries
- PMID: 35382475
- PMCID: PMC8905958
- DOI: 10.1039/d1sc05781b
Recent advances in heterostructured cathodic electrocatalysts for non-aqueous Li-O2 batteries
Abstract
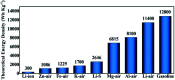
Developing efficient energy storage and conversion applications is vital to address fossil energy depletion and global warming. Li-O2 batteries are one of the most promising devices because of their ultra-high energy density. To overcome their practical difficulties including low specific capacities, high overpotentials, limited rate capability and poor cycle stability, an intensive search for highly efficient electrocatalysts has been performed. Recently, it has been reported that heterostructured catalysts exhibit significantly enhanced activities toward the oxygen reduction reaction and oxygen evolution reaction, and their excellent performance is not only related to the catalyst materials themselves but also the special hetero-interfaces. Herein, an overview focused on the electrocatalytic functions of heterostructured catalysts for non-aqueous Li-O2 batteries is presented by summarizing recent research progress. Reduction mechanisms of Li-O2 batteries are first introduced, followed by a detailed discussion on the typical performance enhancement mechanisms of the heterostructured catalysts with different phases and heterointerfaces, and the various heterostructured catalysts applied in Li-O2 batteries are also intensively discussed. Finally, the existing problems and development perspectives on the heterostructure applications are presented.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures







References
-
- Zhai Y. J. Tong H. Deng J. L. Li G. Y. Hou Y. Zhang R. H. Wang J. Lu Y. Y. Liang K. Chen P. Dang F. Kong B. Energy Storage Materials. 2021;43:391–401. doi: 10.1016/j.ensm.2021.09.017. - DOI
-
- Li Y. Wang L. X. Song A. L. Xia M. R. Li Z. P. Shao G. J. Electrochim. Acta. 2018;268:268–275. doi: 10.1016/j.electacta.2018.02.084. - DOI
-
- Naik K. M. ACS Appl. Energy Mater. 2021;4:1014–1020. doi: 10.1021/acsaem.0c02984. - DOI
-
- Staffell I. Scamman D. Velazquez Abad A. Balcombe P. Dodds P. E. Ekins P. Shah N. S. Ward K. R. Energy Environ. Sci. 2019;12:463–491. doi: 10.1039/C8EE01157E. - DOI
Publication types
LinkOut - more resources
Full Text Sources

