Aptasensors versus immunosensors-Which will prevail?
- PMID: 35382545
- PMCID: PMC8961048
- DOI: 10.1002/elsc.202100148
Aptasensors versus immunosensors-Which will prevail?
Abstract
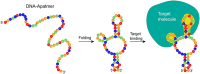
Since the invention of the first biosensors 70 years ago, they have turned into valuable and versatile tools for various applications, ranging from disease diagnosis to environmental monitoring. Traditionally, antibodies have been employed as the capture probes in most biosensors, owing to their innate ability to bind their target with high affinity and specificity, and are still considered as the gold standard. Yet, the resulting immunosensors often suffer from considerable limitations, which are mainly ascribed to the antibody size, conjugation chemistry, stability, and costs. Over the past decade, aptamers have emerged as promising alternative capture probes presenting some advantages over existing constraints of immunosensors, as well as new biosensing concepts. Herein, we review the employment of antibodies and aptamers as capture probes in biosensing platforms, addressing the main aspects of biosensor design and mechanism. We also aim to compare both capture probe classes from theoretical and experimental perspectives. Yet, we highlight that such comparisons are not straightforward, and these two families of capture probes should not be necessarily perceived as competing but rather as complementary. We, thus, elaborate on their combined use in hybrid biosensing schemes benefiting from the advantages of each biorecognition element.
Keywords: antibody; antibody‐aptamer hybrid; aptamer; biosensors; capture probes.
© 2022 The Authors. Engineering in Life Sciences published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors have declared no conflicts of interest.
Figures



References
-
- CDC . Interim Guidance for Antigen Testing for SARS‐CoV‐2 . 2021. June 14th, 2021 [cited 2021 September 10th]; Available from: https://www.cdc.gov/coronavirus/2019‐ncov/lab/resources/antigen‐tests‐gu...
-
- Thévenot DR, Toth K, Durst RA, Wilson GS. Electrochemical biosensors: recommended definitions and classification. Biosens Bioelectron. 2001;16:121‐131. - PubMed
-
- Robertson DL, Joyce GF. Selection in vitro of an RNA enzyme that specifically cleaves single‐stranded DNA. Nature. 1990;344:467‐468. - PubMed
-
- Tuerk C, Gold L. Systematic evolution of ligands by exponential enrichment: RNA ligands to bacteriophage T4 DNA polymerase. Science. 1990;249:505‐510. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous
