A stochastic mathematical model of 4D tumour spheroids with real-time fluorescent cell cycle labelling
- PMID: 35382573
- PMCID: PMC8984298
- DOI: 10.1098/rsif.2021.0903
A stochastic mathematical model of 4D tumour spheroids with real-time fluorescent cell cycle labelling
Abstract
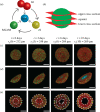
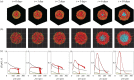
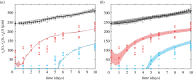
In vitro tumour spheroids have been used to study avascular tumour growth and drug design for over 50 years. Tumour spheroids exhibit heterogeneity within the growing population that is thought to be related to spatial and temporal differences in nutrient availability. The recent development of real-time fluorescent cell cycle imaging allows us to identify the position and cell cycle status of individual cells within the growing spheroid, giving rise to the notion of a four-dimensional (4D) tumour spheroid. We develop the first stochastic individual-based model (IBM) of a 4D tumour spheroid and show that IBM simulation data compares well with experimental data using a primary human melanoma cell line. The IBM provides quantitative information about nutrient availability within the spheroid, which is important because it is difficult to measure these data experimentally.
Keywords: FUCCI; cancer; individual-based model; melanoma; population dynamics.
Conflict of interest statement
We declare we have no competing interests.
Figures





References
-
- Lazzari G, Couvreur P, Mura S. 2017. Multicellular tumor spheroids: a relevant 3D model for the in vitro preclinical investigation of polymer nanomedicines. Polymer Chem. 8, 4947-4969. (10.1039/C7PY00559H) - DOI
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

