The RIO trial: rationale, design, and the role of community involvement in a randomised placebo-controlled trial of antiretroviral therapy plus dual long-acting HIV-specific broadly neutralising antibodies (bNAbs) in participants diagnosed with recent HIV infection-study protocol for a two-stage randomised phase II trial
- PMID: 35382844
- PMCID: PMC8981886
- DOI: 10.1186/s13063-022-06151-w
The RIO trial: rationale, design, and the role of community involvement in a randomised placebo-controlled trial of antiretroviral therapy plus dual long-acting HIV-specific broadly neutralising antibodies (bNAbs) in participants diagnosed with recent HIV infection-study protocol for a two-stage randomised phase II trial
Abstract
Background: Antiretroviral therapy (ART) has led to dramatic improvements in survival for people living with HIV, but is unable to cure infection, or induce viral control off therapy. Designing intervention trials with novel agents with the potential to confer a period of HIV remission without ART remains a key scientific and community goal. We detail the rationale, design, and outcomes of a randomised, placebo-controlled trial of two HIV-specific long-acting broadly neutralising antibodies (bNAbs): 3BNC117-LS and 10-1074-LS, which target CD4 binding site and V3 loop respectively, on post-treatment viral control.
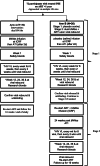
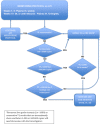
Methods: RIO is a randomised, placebo-controlled, double-blinded prospective phase II study. Eligible individuals will have started ART within 3 months of primary HIV infection and have viral sequences that appear to be sensitive to both bNAbs. It will randomise 72 eligible participants 1:1 to the following arms via a two-stage design. In Stage 1, arm A participants are given dual long-acting (LS-variants) bNAbs infusions, followed by intensively monitored Analytical Treatment Interruption (ATI) (n = 36); in arm B, participants receive placebo infusions followed by ATI. The primary endpoint will be time to viral rebound within 36 weeks after ATI. Upon viral rebound, the participant and researcher are unblinded. Participants in arm A recommence ART and complete the study. Participants in arm B are invited to restart ART and enroll into Stage 2 where they will receive open-label LS bNAbs, followed by a second ATI 24 weeks after. Secondary and exploratory endpoints include adverse events, time to undetectable viraemia after restarting ART, immunological markers, HIV proviral DNA, serum bNAb concentrations in blood, bNAb resistance at viral rebound, and quality of life measures.
Discussion: The two-stage design was determined in collaboration with community involvement. This design allows all participants the option to receive bNAbs. It also tests the hypothesis that bNAbs may drive sustained HIV control beyond the duration of detectable bNAb concentrations. Community representatives were involved at all stages. This included the two-stage design, discussion on the criteria to restart ART, frequency of monitoring visits off ART, and reducing the risk of onward transmission to HIV-negative partners. It also included responding to the challenges of COVID-19.
Trial registration: The protocol is registered on Clinical.
Trials: gov and EudraCT and has approval from UK Ethics and MHRA.
Keywords: Antiretroviral therapy; Broadly neutralising antibodies; HIV; Primary infection; T cell Immunity; Virological remission.
© 2022. The Author(s).
Conflict of interest statement
MJL has received grants and honoraria from Gilead Sciences and Viiv Healthcare not related to this work. Rockefeller University has a patent on 3BNC117 and 10-1074 on which MCN is an inventor. The patent has been licenced to Gilead. All other authors declare that they have no competing interests.
Figures
References
-
- Katz IT, Ryu AE, Onuegbu AG, Psaros C, Weiser SD, Bangsberg DR, et al. Impact of HIV-related stigma on treatment adherence: systematic review and meta-synthesis. J Int AIDS Soc. 2013;16 [cited 2021 Mar 13]. Available from: https://pubmed.ncbi.nlm.nih.gov/24242258/. - PMC - PubMed
-
- Caskey M, Klein F, Nussenzweig MC. Broadly neutralizing anti-HIV-1 monoclonal antibodies in the clinic. Nat Med Nature Publishing Group. 2019;25:547–53 Available from: https://pubmed.ncbi.nlm.nih.gov/30936546/. [cited 2021 Mar 11]. - PMC - PubMed
-
- Walker BD, Yu XG. Unravelling the mechanisms of durable control of HIV-1 [Internet]. Vol. 13, Nature Reviews Immunology. Nat Rev Immunol. 2013:487–98 Available from: https://pubmed.ncbi.nlm.nih.gov/23797064/. [cited 6 Apr 2021]. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials