Reversal of immune-checkpoint inhibitor fulminant myocarditis using personalized-dose-adjusted abatacept and ruxolitinib: proof of concept
- PMID: 35383117
- PMCID: PMC8984056
- DOI: 10.1136/jitc-2022-004699
Reversal of immune-checkpoint inhibitor fulminant myocarditis using personalized-dose-adjusted abatacept and ruxolitinib: proof of concept
Erratum in
-
Correction: Reversal of immune-checkpoint inhibitor fulminant myocarditis using personalized-dose-adjusted abatacept and ruxolitinib: proof of concept.J Immunother Cancer. 2022 May;10(5):e004699corr1. doi: 10.1136/jitc-2022-004699corr1. J Immunother Cancer. 2022. PMID: 35640932 Free PMC article. No abstract available.
Abstract
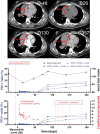
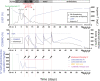
Immune-checkpoint inhibitors (ICI) have revolutionized cancer therapy but are associated with infrequent but lethal myocarditis, for which management remains uncertain. Abatacept, a CTLA-4 fusion protein targeting CD86 on antigen presenting cells and leading to global T-cell anergy, has been described as a potential treatment in individual reports. Yet, abatacept treatment dosage, schedule and optimal combination with other immunosuppressive therapies are unclear. We describe a 25-year-old man who developed pembrolizumab (anti-PD1)-induced myocarditis 14 days after first injection for thymoma treatment, which deteriorated into cardiogenic shock, with sustained ventricular arrhythmia, requiring urgent extracorporeal life support implantation, despite prompt initiation of corticosteroids and mycophenolate-mofetil. Using a strategy of serial measurement ensuring with a target of >80% CD86 receptor occupancy on circulating monocytes, abatacept dose was adjusted and combined with ruxolitinib and methylprednisolone. This strategy resulted in high-dose of abatacept: 60 mg/kg in three doses (20 mg/kg each) within the first 10 days, followed by two doses. Clinical improvement occurred within 7 days, with resolution of systolic cardiac dysfunction, and ventricular arrhythmias resulting in successful discharge from hospital. We reversed a case of nearly lethal ICI-myocarditis, using specific patient-dose adjusted abatacept, which may serve as basis for personalized treatment of patients with severe ICI-adverse events. Trial registration number: NCT04294771.
Keywords: autoimmunity; case reports; immunotherapy.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: Written consent for publication was obtained from the patient. J-ES have participated to advisory boards or consultancy from BMS, Novartis, Banook, AstraZeneca and Beigene. Other authors have nothing to disclose regarding this manuscript. SE received modest consultant fees from AstraZeneca, Amgen, BMS, Banook, Celgene and EISAI. J-ES, JM and YA have patents related to the treatment of ICI related immune adverse events. JM has served on advisory boards for Bristol Myers Squibb, Takeda, Audentes, Deciphera, Janssen, Immuno-Core, Boston Biomedical, Amgen, Myovant, Kurome Therapeutics, Star Therapeutics, ProtinQure, Pharmacyclics, Pfizer, Mallinckrodt Pharmaceuticals, Silverback Therapeutics, Cytokinetics, and AstraZeneca. JM was supported by National Institutes of Health grants (R01HL141466, R01HL155990, and R01HL156021).
Figures
References
-
- Wei SC, Meijers WC, Axelrod ML, et al. . A genetic mouse model recapitulates immune checkpoint inhibitor-associated myocarditis and supports a mechanism-based therapeutic intervention. Cancer Discov 2021;11:614–25. 10.1158/2159-8290.CD-20-0856 - DOI - PMC - PubMed
