Antibody development and disease severity of COVID-19 in non-immunised patients with rheumatic immune-mediated inflammatory diseases: data from a prospective cohort study
- PMID: 35383121
- PMCID: PMC8983412
- DOI: 10.1136/rmdopen-2021-002035
Antibody development and disease severity of COVID-19 in non-immunised patients with rheumatic immune-mediated inflammatory diseases: data from a prospective cohort study
Abstract
Background: Research on the disease severity of COVID-19 in patients with rheumatic immune-mediated inflammatory diseases (IMIDs) has been inconclusive, and long-term prospective data on the development of SARS-CoV-2 antibodies in these patients are lacking.
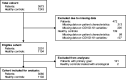
Methods: Adult patients with rheumatic IMIDs from the Amsterdam Rheumatology and Immunology Center, Amsterdam were invited to participate. All patients were asked to recruit their own sex-matched and age-matched control subject. Clinical data were collected via online questionnaires (at baseline, and after 1-4 and 5-9 months of follow-up). Serum samples were collected twice and analysed for the presence of SARS-CoV-2-specific antibodies. Subsequently, IgG titres were quantified in samples with a positive test result.
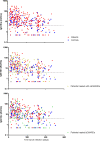
Findings: In total, 3080 consecutive patients and 1102 controls with comparable age and sex distribution were included for analyses. Patients were more frequently hospitalised compared with controls when infected with SARS-CoV-2; 7% vs 0.7% (adjusted OR: 7.33, 95% CI: 0.96 to 55.77). Only treatment with B-cell targeting therapy was independently associated with an increased risk of COVID-19-related hospitalisation (adjusted OR: 14.62, 95% CI: 2.31 to 92.39). IgG antibody titres were higher in hospitalised compared with non-hospitalised patients, and slowly declined with time in similar patterns for patients in all treatment subgroups and controls.
Interpretation: We observed that patients with rheumatic IMIDs, especially those treated with B-cell targeting therapy, were more likely to be hospitalised when infected with SARS-CoV-2. Treatment with conventional synthetic disease-modifying antirheumatic drugs (DMARDs) and biological DMARDs other than B-cell targeting agents is unlikely to have negative effects on the development of long-lasting humoral immunity against SARS-CoV-2.
Keywords: COVID-19; antirheumatic agents; autoimmune diseases; biological therapy; epidemiology.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures
References
-
- Ayala Gutiérrez MDM, Rubio-Rivas M, Romero Gómez C, et al. . Autoimmune diseases and COVID-19 as risk factors for poor outcomes: data on 13,940 hospitalized patients from the Spanish nationwide SEMI-COVID-19 registry. J Clin Med 2021;10. 10.3390/jcm10091844. [Epub ahead of print: 23 04 2021]. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
