Diagnostic performance of screening tools for the detection of obstructive sleep apnea in people living with HIV
- PMID: 35383569
- PMCID: PMC9243280
- DOI: 10.5664/jcsm.9964
Diagnostic performance of screening tools for the detection of obstructive sleep apnea in people living with HIV
Abstract
Study objectives: Many people living with human immunodeficiency virus (PLWH) have undiagnosed obstructive sleep apnea (OSA), which may contribute to commonly reported fatigue and the high cardiovascular disease burden in this population. Our objective was to assess the utility of traditional OSA screening tools (STOP-BANG, Berlin Questionnaire, and Epworth Sleepiness Scale) for detecting OSA in PLWH.
Methods: Adult PLWH were recruited from sleep/ human immunodeficiency virus clinics and the community into a larger clinical trial that included completion of these questionnaires before in-laboratory polysomnography. Discriminatory performance of these screening tools was assessed using area under receiver operating characteristic curves (AUC). The reference standard for the primary analysis was OSA based on an apnea-hypopnea index ≥ 5 events/h using recommended "1A"-criteria (hypopnea with 3% desaturation and/or arousal). Secondary analyses explored acceptable "1B"-criteria (hypopnea with 4% desaturation) and/or higher apnea-hypopnea index cut-offs (≥ 15 events/h).
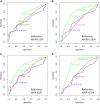
Results: 120 PLWH were included (mean age: 50 ± 11 years; body mass index: 27 ± 4 kg/m2, 84% male) and OSA was diagnosed in 75% using 1A-criteria. In the primary analysis, the discriminatory performance of the 3 screening tools was low (AUCs 0.58 to 0.70) and similar across the tools (P ≥ .14). In secondary analyses, STOP-BANG showed moderate-high discriminatory ability (AUCs 0.77-0.80) and performed significantly better (P ≤ .008) than the Berlin Questionnaire or Epworth Sleepiness Scale (AUCs 0.53-0.62).
Conclusions: OSA was highly prevalent in our cohort of PLWH. Although STOP-BANG could reasonably identify moderate-severe OSA, the tools were not reliable for mild disease. Specifically, the questionnaires perform poorly for PLWH with mild OSA manifesting with arousals, yet such people may be at risk of fatigue/sleepiness and impaired memory consolidation.
Clinical trial registration: Registry: ClinicalTrials.gov; Title: Obstructive Sleep Apnea Endotypes and Impact on Phenotypes of People Living with HIV (PLWH/OSA); Identifier: NCT03575143; URL: https://clinicaltrials.gov/ct2/show/NCT03575143.
Citation: Schmickl CN, Bosompra N-O, DeYoung PN, et al. Diagnostic performance of screening tools for the detection of obstructive sleep apnea in people living with HIV. J Clin Sleep Med. 2022;18(7):1797-1804.
Keywords: HIV; obstructive sleep apnea; people living with HIV; screening.
© 2022 American Academy of Sleep Medicine.
Conflict of interest statement
All authors have seen and approved this manuscript. Dr. Schmickl reports funding from the ATS Foundation (ASPIRE Fellowship), outside the present work. Dr. Orr reports funding from the National Institutes of Health (NIH), as well as advisory board fees from ResMed Inc. outside the present work. Dr. Malhotra is funded by NIH. He reports income related to medical education from Livanova, Corvus, Equillium and Corvus. ResMed provided a philanthropic donation to University of California San Diego (UCSD). Dr. Grant reports funding from NIH and the Wholistic Research Foundation. Dr. Ancoli-Israel is a consultant for Eisai, Biogen, Idorsia, Merck. Dr. Karris reports funding from the NIH, as well as funding to the institution for research by Gilead Sciences and ViiV Healthcare. Dr. Owens reports funding from the NIH, as well as consulting fees from Gwell Health and Nitto Denko Asia, outside the present work. The project described utilized resources from the UCSD Clinical and Translational Research Center, which was supported by the NIH Grant UL1TR000100 of Clinical and Translational Science Awards (CTSA) funding prior to August 13, 2015 and Grant UL1TR001442 of CTSA funding beginning August 13, 2015 and beyond. This publication was also supported by the San Diego Center for AIDS Research (SD CFAR), an NIH-funded program (P30 AI036214), which is supported by the following NIH Institutes and Centers: National Institute of Allergy and Infectious Diseases, National Cancer Institute, National Heart, Lung, and Blood Institute, National Institute on Aging, National Institute of Child Health and Human Development, National Institute on Drug Abuse, National Institute of Dental and Craniofacial Research, National Institute of Diabetes and Digestive and Kidney Diseases, National Institute of General Medical Sciences, National Institute of Mental Health, Fogarty International Center (FIC) and Office of AIDS Research (OAR). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Figures
References
-
- Payne BA , Hateley CL , Ong EL , et al. . HIV-associated fatigue in the era of highly active antiretroviral therapy: Novel biological mechanisms? HIV Med. 2013. ; 14 ( 4 ): 247 – 251 . - PubMed
-
- Allavena C , Guimard T , Billaud E , et al. . COREVIH-Pays de la Loire Troubles du Sommeil Study Group . Prevalence and risk factors of sleep disturbance in a large HIV-infected adult population . AIDS Behav. 2016. ; 20 ( 2 ): 339 – 344 . - PubMed

