Vitamin D supplementation in chronic obstructive pulmonary disease patients with low serum vitamin D: a randomized controlled trial
- PMID: 35383823
- PMCID: PMC9348978
- DOI: 10.1093/ajcn/nqac083
Vitamin D supplementation in chronic obstructive pulmonary disease patients with low serum vitamin D: a randomized controlled trial
Abstract
Background: Vitamin D deficiency is frequently found in patients with chronic obstructive pulmonary disease (COPD). Vitamin D has antimicrobial, anti-inflammatory, and immunomodulatory effects. Therefore, supplementation may prevent COPD exacerbations, particularly in deficient patients.
Objectives: We aimed to assess the effect of vitamin D supplementation on exacerbation rate in vitamin D-deficient patients with COPD.
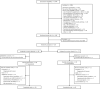
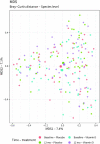
Methods: We performed a multicenter, double-blind, randomized controlled trial. COPD patients with ≥1 exacerbations in the preceding year and a vitamin D deficiency (15-50 nmol/L) were randomly allocated in a 1:1 ratio to receive either 16,800 International Units (IU) vitamin D3 or placebo once a week during 1 y. Primary outcome of the study was exacerbation rate. Secondary outcomes included time to first and second exacerbations, time to first and second hospitalizations, use of antibiotics and corticosteroids, pulmonary function, maximal respiratory mouth pressure, physical performance, skeletal muscle strength, systemic inflammatory markers, nasal microbiota composition, and quality of life.
Results: The intention-to-treat population consisted of 155 participants. Mean ± SD serum 25-hydroxyvitamin D [25(OH)D] concentration after 1 y was 112 ± 34 nmol/L in the vitamin D group, compared with 42 ± 17 nmol/L in the placebo group. Vitamin D supplementation did not affect exacerbation rate [incidence rate ratio (IRR): 0.90; 95% CI: 0.67, 1.21]. In a prespecified subgroup analysis in participants with 25(OH)D concentrations of 15-25 nmol/L (n = 31), no effect of vitamin D supplementation was found (IRR: 0.91; 95% CI: 0.43, 1.93). No relevant differences were found between the intervention and placebo groups in terms of secondary outcomes.
Conclusions: Vitamin D supplementation did not reduce exacerbation rate in COPD patients with a vitamin D deficiency.This trial was registered at clinicaltrials.gov as NCT02122627.
Keywords: chronic obstructive pulmonary disease; exacerbation rate; muscle strength; physical function; pulmonary function; vitamin D.
© The Author(s) 2022. Published by Oxford University Press on behalf of the American Society for Nutrition.
Figures
References
-
- Black PN, Scragg R. Relationship between serum 25-hydroxyvitamin D and pulmonary function in the Third National Health and Nutrition Examination Survey. Chest. 2005;128(6):3792–8. - PubMed
-
- Jolliffe DA, James WY, Hooper RL, Barnes NC, Greiller CL, Islam Ket al. . Prevalence, determinants and clinical correlates of vitamin D deficiency in patients with Chronic Obstructive Pulmonary Disease in London, UK. J Steroid Biochem Mol Biol. 2018;175:138–45. - PubMed
-
- Beaudart C, Buckinx F, Rabenda V, Gillain S, Cavalier E, Slomian Jet al. . The effects of vitamin D on skeletal muscle strength, muscle mass, and muscle power: a systematic review and meta-analysis of randomized controlled trials. J Clin Endocrinol Metab. 2014;99(11):4336–45. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

