Conditional survival and long-term efficacy with nivolumab plus ipilimumab versus sunitinib in patients with advanced renal cell carcinoma
- PMID: 35383908
- PMCID: PMC9543316
- DOI: 10.1002/cncr.34180
Conditional survival and long-term efficacy with nivolumab plus ipilimumab versus sunitinib in patients with advanced renal cell carcinoma
Abstract
Background: Conditional survival estimates provide critical prognostic information for patients with advanced renal cell carcinoma (aRCC). Efficacy, safety, and conditional survival outcomes were assessed in CheckMate 214 (ClinicalTrials.gov identifier NCT02231749) with a minimum follow-up of 5 years.
Methods: Patients with untreated aRCC were randomized to receive nivolumab (NIVO) (3 mg/kg) plus ipilimumab (IPI) (1 mg/kg) every 3 weeks for 4 cycles, then either NIVO monotherapy or sunitinib (SUN) (50 mg) daily (four 6-week cycles). Efficacy was assessed in intent-to-treat, International Metastatic Renal Cell Carcinoma Database Consortium intermediate-risk/poor-risk, and favorable-risk populations. Conditional survival outcomes (the probability of remaining alive, progression free, or in response 2 years beyond a specified landmark) were analyzed.
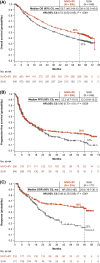
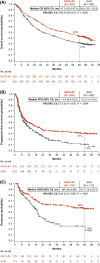
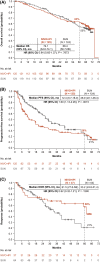
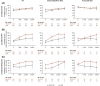
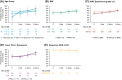
Results: The median follow-up was 67.7 months; overall survival (median, 55.7 vs 38.4 months; hazard ratio, 0.72), progression-free survival (median, 12.3 vs 12.3 months; hazard ratio, 0.86), and objective response (39.3% vs 32.4%) benefits were maintained with NIVO+IPI versus SUN, respectively, in intent-to-treat patients (N = 550 vs 546). Point estimates for 2-year conditional overall survival beyond the 3-year landmark were higher with NIVO+IPI versus SUN (intent-to-treat patients, 81% vs 72%; intermediate-risk/poor-risk patients, 79% vs 72%; favorable-risk patients, 85% vs 72%). Conditional progression-free survival and response point estimates were also higher beyond 3 years with NIVO+IPI. Point estimates for conditional overall survival were higher or remained steady at each subsequent year of survival with NIVO+IPI in patients stratified by tumor programmed death ligand 1 expression, grade ≥3 immune-mediated adverse event experience, body mass index, and age.
Conclusions: Durable clinical benefits were observed with NIVO+IPI versus SUN at 5 years, the longest phase 3 follow-up for a first-line checkpoint inhibitor-based combination in patients with aRCC. Conditional estimates indicate that most patients who remained alive or in response with NIVO+IPI at 3 years remained so at 5 years.
Keywords: CheckMate 214; advanced renal cell carcinoma; dual checkpoint inhibition; durable response; long-term follow-up; nivolumab plus ipilimumab; phase 3.
© 2022 The Authors. Cancer published by Wiley Periodicals LLC on behalf of American Cancer Society.
Conflict of interest statement
Robert J. Motzer reports institutional grants or contracts from Aveo Pharmaceuticals, Bristol Myers Squibb (BMS), Eisai, Exelixis Inc, Genentech/Roche, Merck, Pfizer; and personal fees for advisory board participation from AstraZeneca, Aveo Pharmaceuticals, Eisai, EMD Serono, Exelixis Inc, Genentech/Roche, Incyte, Lilly Oncology, Merck, Novartis, and Pfizer, all outside the submitted work. David F. McDermott reports institutional research grants from Alkermes Inc, BMS, Genentech/Roche, Merck, Novartis, Peloton Therapeutics, and Prometheus Laboratories; consulting or advisory fees from Alkermes Inc, Array BioPharma, Aveo Pharmaceuticals, BMS, Calithera Biosciences, Checkmate Pharmaceuticals, CRISPR Therapeutics, Eisai, Eli Lilly and Company, EMD Serono, Exelixis Inc, Genentech/Roche, Iovance, Johnson & Johnson, Jounce Therapeutics, Merck, Novartis, Peloton Therapeutics, Pfizer, Synthekine Inc, Werewolf Therapeutics, and X4 Pharma; and other fees from the Beth Israel Deaconess Medical Center, all outside the submitted work. Bernard Escudier reports personal fees from Aveo Pharmaceuticals, BMS, Eisai, Ipsen, Oncorena, and Pfizer; honoraria from BMS, Ipsen, Oncorena, and Pfizer; and support for meetings and/or travel accommodations/expenses from BMS, Ipsen, and MSD, all outside the submitted work. Mauricio Burotto reports honoraria from AstraZeneca, BMS, Merck, Novartis, and Roche; and participation on a data safety monitoring board at AstraZeneca, BMS, Merck, and Roche, all outside the submitted work. Toni K. Choueiri reports support in part by grants from the National Cancer Institute to the Dana‐Farber/Harvard Specialized Program of Research Excellence (2P50CA101942‐16) and Program 5P30CA006516‐56, the Kohlberg Chair at Harvard Medical School, and the Trust Family, Michael Brigham, and Loker Pinard Funds for Kidney Cancer Research at Dana Farber Cancer Institute; institutional research funding for clinical trials from the Alliance Cooperative Group, AstraZeneca, Aveo Pharmaceuticals, Bayer, BMS, Eisai, EMD Serono, Exelixis Inc, Eli Lilly and Company, GlaxoSmithKline (GSK), Merck, Nikang Therapeutics, Novartis, Orien, Peloton, Pfizer, Roche, Sanofi‐Aventis, and Takeda; royalties/licensing fees from Up‐To‐Date (online textbook); personal fees from Analysis Group, Aptitude Health, AstraZeneca, Aravive, Aveo Pharmaceuticals, Bayer, BMS, Calithera Biosciences, Circle Pharma, Eisai, Eli Lilly and Company, EMD Serono, Exelixis Inc, GSK, IQVIA, Infinity Pharmaceuticals, Ipsen, Kanaph, Merck, Nikang Therapeutics, Novartis, Nuscan, Pfizer, Roche, Sanofi/Aventis, Surface Oncology, Takeda, Tempest, Up‐To‐Date, and continuing medical education events (PeerView, PER, MJH Life Sciences, Research to Practice, France Foundation, Springer, WebMed, ASiM Ce, and Caribou Publishing); honoraria from Advent Health, the American Society of Clinical Oncology (ASCO)‐Society for the Immunotherapy of Cancer, ASiM Ce, CancerNet, Ipsen, The University of Texas MD Anderson Cancer Center, MJH Life Sciences, NAMC, PeerView, PER, Research to Practice, Springer, and WebMD; participation on a data safety monitoring board at Aravive; a leadership or fiduciary role in KidneyCan (unpaid), ASCO, the European Society for Medical Oncology, the National Comprehensive Cancer Network, and the Genitourinary Steering Committee of the National Cancer Institute; stock ownership in Nuscan, Osel, Pionyr, and Tempest; and principal investigator fees (institutional) from GSK, Roche, and Surface Oncology, all outside the submitted work. Hans J. Hammers reports clinical research funding from Aravive, BMS, Merck, and Surface Oncology; personal fees from ARMO Biosciences, Aveo Pharmaceuticals, BMS, Corvus, Merck, Pfizer, and Surface Oncology; and receipt of drugs for clinical trials from BMS, all outside the submitted work. Philippe Barthélémy reports personal fees from Amgen, Astellas Pharma, Bayer, BMS, Eisai, Ipsen, Janssen‐Cilag, Merck, Merck Sharp & Dohme (MSD), Novartis, Pfizer, and Roche; honoraria from Amgen, Astellas Pharma, AstraZeneca, Bayer, BMS, Ipsen, Janssen‐Cilag, Merck, Novartis, Pfizer, and Seagen; and support for travel accommodations/expenses from BMS, Ipsen, Janssen‐Cilag, Merck, MSD, Novartis, and Pfizer, all outside the submitted work. Elizabeth R. Plimack reports grants for clinical research from Astellas Pharma, BMS, Genentech, and Merck; personal fees from Astellas Pharma, AstraZeneca, Aveo Pharmaceuticals, BMS, Calithera Biosciences, Flatiron, Genentech, Infinity Pharmaceuticals, Janssen, MEI Pharma, Merck, Pfizer, Regeneron, and Seattle Genetics; honoraria from ACHL, Clinical Care Options, Creative Educational Solutions, CUA, Fox Chase Cancer Center, Georgetown University, American Society of Clinical Oncology Genitourinary Cancers Symposium (GU ASCO), JADPRO Bladder, Medscape, Mount Sinai, the National Comprehensive Cancer Network, Ohio State University, OncLive, Peak Medicals GmbH, PER, Research to Practice, Spire Learning, Total CME, the University of Hawaii, and the University of Pennsylvania; has applied for a patent for Screening Muscle Invasive Bladder Cancer Patients for Neoadjuvant Chemotherapy Responsiveness (US Patent Application No. 14/588,503); participates on a data safety monitoring board or advisory board for AstraZeneca, Infinity Pharmaceuticals, and Pfizer; and serves on the ASCO board of directors and the Bladder Cancer Advocacy Network Scientific Advisory Board, all outside the submitted work. Camillo Porta reports personal fees from Angelini, AstraZeneca, BMS, Eisai, EUSA Pharma, General Electric, Ipsen, Janssen, Merck, MSD, and Novartis; honoraria from Angelini, AstraZeneca, BMS, Eisai Medical Research, EUSA Pharma, General Electric, Ipsen, Janssen, Merck, MSD, Novartis, and Pfizer; fees for expert testimony from EUSA Pharma and Pfizer; steering committee member fees from BMS, Eisai, and EUSA Pharma; and support for travel accommodations/expenses from Roche, all outside the submitted work. Saby George reports institutional research funding from Aravive, Agensys, Aveo Pharmaceuticals, Bayer, BMS, Calithera Biosciences, Corvus Pharmaceuticals, Eisai, Exelixis Inc, Gilead, Merck, Novartis, Pfizer, Seattle Genetics, and Surface Oncology; personal fees from Aveo Pharmaceuticals, Bayer, BMS, Corvus Pharmaceuticals, Eisai, EMD Serono, Exelixis Inc, Merck, Immunomedics, Pfizer, QED Therapeutics, Sanofi, and Seattle Genetics; and honoraria from Aptitude Health, Curio Science, and DAVA Oncology, all outside the submitted work. Thomas Powles reports institutional research grants from Astellas Pharma, AstraZeneca, BMS, Eisai, Exelixis Inc, Ipsen, Johnson & Johnson, Merck, Merck Serono, MSD, Novartis, Pfizer, Roche, and Seattle Genetics; personal fees from Astellas Pharma, AstraZeneca, BMS, Eisai, Exelixis Inc, Incyte, Ipsen, Johnson & Johnson, Merck, Merck Serono, MSD, Novartis, Pfizer, Roche, and Seattle Genetics; and support for travel accommodations/expenses from Ipsen, MSD, Pfizer, and Roche, all outside the submitted work. Frede Donskov reports institutional research grants from Ipsen, MSD, and Pfizer outside the submitted work. Howard Gurney reports honoraria from BMS, Ipsen, Merck, and Pfizer; and participation on advisory boards for AstraZeneca, BMS, Ipsen, Janssen, Merck, MSD, and Pfizer, all outside the submitted work. Christian K. Kollmannsberger reports personal fees from Astellas Pharma, Bayer, BMS, Eisai, Ipsen, Janssen, Merck, and Pfizer outside the submitted work. Marc‐Oliver Grimm reports institutional research funding as a local/coordinating principal investigator from BMS, Intuitive Surgical, and Novartis; support for travel/accommodations from AstraZeneca, Ipsen, Merck, and Pfizer; participation on a data safety monitoring board or advisory board for Astellas, AstraZeneca, Bayer, BMS, Eisai, EUSA Pharma, Ipsen, Janssen‐Cilag, Merck Serono, MSD, Roche, and Takeda; honoraria from Astellas, AstraZeneca, BMS, EUSA Pharma, Ipsen, Janssen, Merck Serono, MSD, Oncinfo, and Pfizer; and is a member of the board of directors of Deutsche Gesellschaft fur Urologie, all outside the submitted work. Carlos Barrios reports institutional research funding from AB Science, AbbVie, Abraxis Biosciences, Amgen, Asana Biosciences, Astellas Pharma, AstraZeneca, BioMarin, BMS, Boehringer Ingelheim, Celgene, Clinica Atlantas, Covance, Daiichi Sankyo, GSK, Eli Lilly and Company, Exelixis Inc, Halozyme, ImClone Systems, INC Research, inVentiv Health, Janssen, LEO Pharma, Medivation, Merck KGaA, Merrimack, Millennium, Mylan, Novartis, Pfizer, PharmaMar, Polyphor, Roche/Genentech, Sanofi, Shanghai Henlius Biotech, and Taiho Pharmaceutical; consulting or advisory fees from AstraZeneca, Boehringer Ingelheim, Eli Lilly and Company, Eisai, GSK, Libbs, MSD Oncology, Novartis, Pfizer, Roche/Genentech, and United Medical; honoraria from AstraZeneca, Bayer, Boehringer Ingelheim, Eisai, Eli Lilly and Company, GSK, MSD, Novartis, Pfizer, Roche/Genentech, Sanofi, and Zodiac Pharma; participation on an independent data monitoring committee for Roche/Genentech; travel accommodations/expenses from AstraZeneca, BMS Brazil, Eli Lilly and Company, MSD Oncology, Novartis, Pfizer, and Roche/Genentech; and stock ownership in Biomark, MEDSIR, and Tummi, all outside the submitted work. Yoshihiko Tomita reports institutional research grants from Astellas Pharma, Ono Pharmaceutical, and Takeda; and honoraria from Astellas Pharma, BMS, Ono Pharmaceutical, and Pfizer, all outside the submitted work. Daniel Castellano reports institutional research grants from Janssen Oncology; and personal fees from Astellas Pharma, AstraZeneca, Bayer, BMS, Boehringer Ingelheim, Eli Lilly and Company, Ipsen, Janssen Oncology, MSD Oncology, Novartis, Pfizer, Pierre Fabre, Roche/Genentech, and Sanofi, alloutside the submitted work. Viktor Grünwald reports institutional research grants from BMS, Ipsen, MSD, and Pfizer; personal fees from BMS, Eisai, EUSA Pharma, Ipsen, Janssen‐Cilag, Merck Serono, MSD, Nanobiotix, Pfizer, and Roche; honoraria from Apogepha, AstraZeneca, BMS, Eisai, EUSA Pharma, Ipsen, Janssen‐Cilag, Merck Serono, MSD, Pfizer, and Roche; support for travel/accommodations from AstraZeneca, BMS, and Pfizer; is on the faculty of the European Society for Medical Oncology; owns stock in AstraZeneca, BMS, GenMab, MSD, and Seattle Genetics; and is a member of the BMS, Ipsen, and Novartis steering committees, all outside the submitted work. Brian I. Rini reports institutional research funding from Aravive, Arrowhead, AstraZeneca, BMS, Merck, and Pfizer; grants from Aravive, Arrowhead Pharmaceuticals, AstraZeneca, BMS, Dragonfly Therapeutics, Exelixis Inc, Hoffmann‐La Roche, Immunomedics, Incyte, Janssen, Merck, Mirati Therapeutics, Pfizer, Seattle Genetics, and Surface Oncology; personal fees from 3DMedicines, Alkermes, Aravive, Arrowhead, Aveo Pharmaceuticals, BMS, Compugen, Corvus, Eisai, Genentech/Roche, GSK, Merck, Nikang Therapeutics, Pfizer, Shionogi, Surface Oncology, and Synthorx; support for travel/accommodations from Merck and Pfizer; participation on an AstraZeneca data safety monitoring board or advisory board; and owns stock in PTC Therapeutics, all outside the submitted work. M. Brent McHenry is an employee of BMS and owns stock in the company. Chung‐Wei Lee is an employee of BMS and owns stock in the company. Jennifer McCarthy is an employee of BMS. Flavia Ejzykowicz is an employee of BMS and owns stock in the company. Nizar M. Tannir reports institutional funding for clinical trials from Arrowhead, BMS, Calithera Biosciences, Eisai, Nektar Therapeutics, and Novartis; personal fees or honoraria from BMS, Calithera Bioscience, Eisai, Eli Lilly and Company, Exelixis Inc, Ipsen, MSD, Nektar Therapeutics, Novartis, Oncorena, Pfizer, and Surface Oncology; participation as a member of the US Renal Cell Carcinoma Advisory Board Committee; and owns stock in Amgen, Arcturus, Arcus, Bellus Health, BioCryst Pharmaceuticals, Corvus Pharmaceuticals, First Trust Amex Biotech, Johnson & Johnson, Merck, Nuvation Bio Inc Cl A (NUVB), Revolution Medicines, Spdr S&P Pharmaceuticals ETF, Surface Oncology, Vanguard Health Care ETF, and Xencor, all outside the submitted work.
Figures

Comment in
-
Setting a new standard for long-term survival in metastatic kidney cancer.Cancer. 2022 Jun 1;128(11):2058-2060. doi: 10.1002/cncr.34177. Epub 2022 Apr 5. Cancer. 2022. PMID: 35383907 No abstract available.
References
-
- Choueiri TK, Motzer RJ. Systemic therapy for metastatic renal‐cell carcinoma. N Engl J Med. 2017;376:354‐366. - PubMed
-
- Rini BI, Plimack ER, Stus V, et al. Pembrolizumab plus axitinib versus sunitinib for advanced renal‐cell carcinoma. N Engl J Med. 2019;380:1116‐1127. - PubMed
-
- Hall JP, Zanotti G, Kim R, et al. Treatment patterns, outcomes and clinical characteristics in advanced renal cell carcinoma: a real‐world US study. Future Oncol. 2020;16:3045‐3060. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

