Nonlinear Population Pharmacokinetics of Anifrolumab in Healthy Volunteers and Patients With Systemic Lupus Erythematosus
- PMID: 35383948
- PMCID: PMC9540432
- DOI: 10.1002/jcph.2055
Nonlinear Population Pharmacokinetics of Anifrolumab in Healthy Volunteers and Patients With Systemic Lupus Erythematosus
Abstract
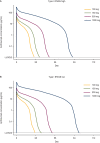
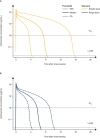
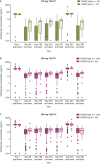
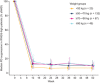
We characterized the population pharmacokinetics of anifrolumab, a type I interferon receptor-blocking antibody. Pharmacokinetic data were analyzed from the anifrolumab (intravenous [IV], every 4 weeks) arms from 5 clinical trials in patients with systemic lupus erythematosus (SLE) (n = 664) and healthy volunteers (n = 6). Population pharmacokinetic modeling was performed using a 2-compartment model with parallel linear and nonlinear elimination pathways. The impact of covariates (demographics, interferon gene signature [IFNGS, high/low], disease characteristics, renal/hepatic function, SLE medications, and antidrug antibodies) on pharmacokinetics was evaluated. Time-varying clearance (CL) was characterized using an empirical sigmoidal time-dependent function. Anifrolumab exposure increased more than dose-proportionally from 100 to 1000 mg IV every 4 weeks. Based on population pharmacokinetics modeling, the baseline median linear CL was 0.193 L/day in IFNGS-high patients and 0.153 L/day in IFNGS-low/healthy volunteers. After a year, median anifrolumab linear CL decreased by 8.4% from baseline. Body weight and IFNGS were significant pharmacokinetic covariates, whereas age, sex, race, disease activity, SLE medications, and presence of antidrug antibodies had no significant effect on anifrolumab pharmacokinetics. Anifrolumab at a concentration of 300 mg IV every 4 weeks was predicted to be below the lower limit of quantitation in 95% of patients ≈10 weeks after a single dose and ≈16 weeks after stopping dosing at steady state. To conclude, anifrolumab exhibited nonlinear pharmacokinetics and time-varying linear CL; doses ≥300 mg IV every 4 weeks provided sustained anifrolumab concentrations. This study provides further evidence to support the use of anifrolumab 300 mg IV every 4 weeks in patients with moderate to severe SLE.
Keywords: drug development; modeling and simulation; pharmacodynamics; population pharmacokinetics; rheumatology.
© 2022 The Authors. The Journal of Clinical Pharmacology published by Wiley Periodicals LLC on behalf of American College of Clinical Pharmacology.
Conflict of interest statement
J.A., R.T., W.I.W., and W.T. are employees of and own stocks of AstraZeneca. D.K. and T.M. are current employees at Genentech‐Roche, former employees of AstraZeneca, and own/owned stocks of AstraZeneca. L.R. is a current employee of Exelixis, former employee of AstraZeneca, and own/owned stocks of AstraZeneca. Y.L.C. is a current employee of Seagen, a former employee of AstraZeneca, and owns stocks of AstraZeneca.
Figures

References
-
- Bengtsson AA, Rönnblom L. Role of interferons in SLE. Best Pract Res Clin Rheumatol. 2017;31(3):415‐428. - PubMed
-
- Feng X, Wu H, Grossman JM, et al. Association of increased interferon‐inducible gene expression with disease activity and lupus nephritis in patients with systemic lupus erythematosus. Arthritis Rheum. 2006;54(9):2951‐2962. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

