Efficiency assessment of irrigation as an alternative method for improving the regenerative potential of non-healing wounds
- PMID: 35384136
- PMCID: PMC9321893
- DOI: 10.1111/wrr.13013
Efficiency assessment of irrigation as an alternative method for improving the regenerative potential of non-healing wounds
Abstract
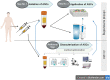
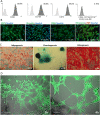
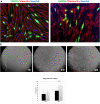
The application of mesenchymal stem/stromal cells (MSC) in regenerative medicine offers hope for the effective treatment of incurable or difficult-to-heal diseases. However, it requires the development of unified protocols for both safe and efficient cell acquisition and clinical usage. The therapeutic effect of fat grafts (containing stem cells) in non-healing wounds has been discussed in previous studies, although the application requires local or general anaesthesia. The treatment of MSC derived from adipose tissue (ASC) could be a less invasive method, and efficient delivery could lead to more favourable outcomes, which should encourage clinicians to use such therapeutic approaches more frequently. Therefore, the aim of this study was to optimise the methods of ASC isolation, culture and administration while maintaining their high survival, proliferation and colonisation potential. The ASC were isolated by an enzymatic method and were characterised according to International Society for Cellular Therapy and International Federation for Adipose Therapeutics and Science guidelines. To assess the opportunity to obtain a sufficient number of cells for transplantation, long-term cell cultures in two oxygen concentrations (5% vs. 21%) were conducted. For these cultures, the population doubling time, the cumulative time for cell population doublings and the rate of cell senescence were estimated. In a developed and pre-defined protocol, ASC can be efficiently cultured at physiological oxygen concentrations (5%), which leads to faster proliferation and slower cell senescence. Subsequently, to select the optimal and minimally invasive methods of ASC transplantation, direct cell application with an irrigator or with skin dressings was analysed. Our results confirmed that both the presented methods of cell application allow for the safe delivery of isolated ASC into wounds without losing their vitality. Cells propagated in the described conditions and applied in non-invasive cell application (with an irrigation system and dressings) to treat chronic wounds can be a potential alternative or supplement to more invasive clinical approaches.
Keywords: cell therapy; irrigation; mesenchymal stem/stromal cells; non-healing wounds.
© 2022 The Authors. Wound Repair and Regeneration published by Wiley Periodicals LLC on behalf of The Wound Healing Society.
Conflict of interest statement
The authors declare that there are no competing financial interests or conflicts of interest regarding the publication of this paper.
Figures




References
-
- Jiang D, Qi Y, Walker NG, et al. The effect of adipose tissue derived MSCs delivered by a chemically defined carrier on full‐thickness cutaneous wound healing. Biomaterials. 2013;34(10):2501‐2515. - PubMed
-
- Wilson WR, Hay MP. Targeting hypoxia in cancer therapy. Nat Rev Cancer. 2011;11(6):393‐410. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

