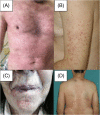
Cutaneous side effects and types of dermatological reactions in metastatic melanoma patients treated by immunotherapies or targeted therapies: A retrospective single center study
- PMID: 35384181
- PMCID: PMC9287008
- DOI: 10.1111/dth.15492
Cutaneous side effects and types of dermatological reactions in metastatic melanoma patients treated by immunotherapies or targeted therapies: A retrospective single center study
Abstract
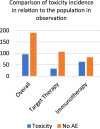
Immunotherapy and target therapy have revolutionized treatment of stage III/IV melanoma. Both treatments show a favorable toxicity profile even if cutaneous adverse events (AEs) are frequent (30%-40% of cases). This is a retrospective single center cohort study that included patients with stage IV or inoperable stage III metastatic melanoma (AJCC 8th) who received BRAFi + MEKi therapy or immunotherapy with Checkpoint inhibitors. All cutaneous AEs were ascertained by a dermatologist based on clinical and histological findings. The primary outcome was to provide a detailed clinical dermatological classification of cutaneous adverse events and an evaluation of the incidence of skin toxicity in the two arms of therapy (immunotherapy and target therapy). A total of 286 patients with stages III-IV metastatic melanoma were included: 146 received immunotherapy and 140 target therapy. In the immunotherapy cohort, 63 (43.1%) cutaneous reactions were observed while 33 skin reactions (23.6%) were identified in patients treated with target therapy. All the skin toxicities observed were grade I, excepted four cases: an erythema multiforme-like eruption, a grade III psoriasis and two grade III maculopapular rashes. Immunotherapy in older age resulted statistically related to skin toxicities (p = 0.011), meanly in metastatic setting (p = 0.011). Cumulative incidence of skin toxicities was 65.63% in immunotherapy cohort (p = 0.001). Also multivariate logistic regression shows a significant association between skin adverse events and immunotherapy (odds ratio [OR] = 0.50; 95% confidence interval [CI]: 0.29-0.85, p: 0.01) and between cutaneous AEs and metastatic setting (OR = 1.97; 95% CI: 1.04-3.74, p: 0.04). We have also shown that as the age of initiation of therapy increases the probability of developing skin toxicity grows. However, stratifying by type of therapies the effect of age persists only in immunotherapy (OD: 1.04; CI: 1.01-1.06; p: 0.04) while for target therapy age does not affect the onset of skin toxicity (OD 1.01; CI 0.98-1.04; p = 0.42). No differences were shown between patients on target therapy and immunotherapy regarding gender. Patients were also evaluated regarding concomitant therapies and seems that Levotyroxine may be involved in AEs during immunotherapy treatment. More studies are needed to deepen this aspect, also considering the medical history and diverse drug associations. Cutaneous adverse events are characterized by heterogeneous manifestations, are more often seen in patients on immunotherapy and dermatologists can play a crucial role in multidisciplinary care.
Keywords: advanced melanoma; adverse events; cutaneous toxicity; immunotherapy; target therapy.
© 2022 The Authors. Dermatologic Therapy published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Using a cancer registry to capture signals of adverse events following immune and targeted therapy for melanoma.Int J Clin Pharm. 2018 Aug;40(4):852-861. doi: 10.1007/s11096-018-0665-1. Epub 2018 Jun 2. Int J Clin Pharm. 2018. PMID: 29860707
-
Cutaneous Toxicities of Advanced Treatment for Cutaneous Melanoma: A Prospective Study from a Single-Center Institution.Cancers (Basel). 2024 Oct 30;16(21):3679. doi: 10.3390/cancers16213679. Cancers (Basel). 2024. PMID: 39518117 Free PMC article.
-
Cutaneous adverse reactions in B-RAF positive metastatic melanoma following sequential treatment with B-RAF/MEK inhibitors and immune checkpoint blockade or vice versa. A single-institutional case-series.J Immunother Cancer. 2019 Jan 8;7(1):4. doi: 10.1186/s40425-018-0475-y. J Immunother Cancer. 2019. PMID: 30621779 Free PMC article.
-
Cutaneous Complications of Targeted Melanoma Therapy.Curr Treat Options Oncol. 2016 Nov;17(11):57. doi: 10.1007/s11864-016-0434-0. Curr Treat Options Oncol. 2016. PMID: 27645330 Review.
-
Cutaneous Adverse Events of Immune Checkpoint Inhibitors: A Summarized Overview.Curr Drug Saf. 2019;14(1):14-20. doi: 10.2174/1574886313666180730114309. Curr Drug Saf. 2019. PMID: 30058498 Review.
Cited by
-
Editorial: Cutaneous immunology.Front Med (Lausanne). 2023 Aug 24;10:1275332. doi: 10.3389/fmed.2023.1275332. eCollection 2023. Front Med (Lausanne). 2023. PMID: 37692788 Free PMC article. No abstract available.
-
A novel risk model based on anoikis: Predicting prognosis and immune infiltration in cutaneous melanoma.Front Pharmacol. 2023 Jan 16;13:1090857. doi: 10.3389/fphar.2022.1090857. eCollection 2022. Front Pharmacol. 2023. PMID: 36726781 Free PMC article.
-
Impact of Immunosenescence on Immune-Related Adverse Events in Elderly Patients With Cancer.Aging Med (Milton). 2025 Feb 26;8(1):e70000. doi: 10.1002/agm2.70000. eCollection 2025 Feb. Aging Med (Milton). 2025. PMID: 40012861 Free PMC article.
-
The fusion of light and immunity: Advancements in photoimmunotherapy for melanoma.Photochem Photobiol. 2024 Jul-Aug;100(4):910-922. doi: 10.1111/php.13951. Epub 2024 Apr 16. Photochem Photobiol. 2024. PMID: 38623955 Free PMC article. Review.
-
Skin Reactions and Other Underappreciated Dermatologic Side Effects of Cancer Therapies.Curr Treat Options Oncol. 2025 Aug;26(8):726-753. doi: 10.1007/s11864-025-01333-5. Epub 2025 Aug 14. Curr Treat Options Oncol. 2025. PMID: 40813519 Review.
References
-
- Khan KH, Goody RB, Hameed H, Jalil A, Coyle VM, McAleer JJ. Metastatic melanoma: a regional review and future directions. Tumori. 2012;98(5):575‐580. - PubMed
-
- Testori AAE, Ribero S, Indini A, Mandalà M. Adjuvant treatment of melanoma: recent developments and future perspectives. Am J Clin Dermatol. 2019;20(6):817‐827. - PubMed
-
- Lacouture ME, Sibaud V, Gerber PA, et al. Prevention and management of dermatological toxicities related to anticancer agents: ESMO clinical practice guidelines. Ann Oncol. 2021;32(2):157‐170. - PubMed
-
- Gnanendran SS, Turner LM, Miller JA, Hwang SJE, Miller AC. Cutaneous adverse events of anti‐PD‐1 therapy and BRAF inhibitors. Curr Treat Options Oncol. 2020;21(4):29. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical