Testis-specific hnRNP is expressed in colorectal cancer cells and accelerates cell growth mediating ZDHHC11 mRNA stabilization
- PMID: 35384384
- PMCID: PMC9554453
- DOI: 10.1002/cam4.4738
Testis-specific hnRNP is expressed in colorectal cancer cells and accelerates cell growth mediating ZDHHC11 mRNA stabilization
Abstract
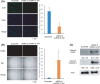
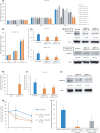
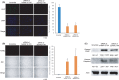
Various heterogeneous nuclear ribonucleoproteins (hnRNPs) have been reported to be associated with cancer cell growth. However, it remains unclear whether hnRNP G-T, which is specifically expressed in the testis, is expressed in tumor cells, and whether hnRNP G-T expressed in colorectal cancer (CRC) cells is associated with tumor progression. We herein report that hnRNP G-T promoted cancer cell growth and stabilized mRNA of ZDHHC11 in CRC. The cell growth was inhibited by transfection of siRNA of hnRNP G-T in cancer cells, but not in non-cancerous epithelial cells. The tumor promotive effect of hnRNP G-T was confirmed in an HCT116 transplanted mouse model. RT-PCR and western blotting indicated the augmentation of hnRNP G-T in CRC in comparison to non-cancerous cells. The downregulation of hnRNP G-T inhibited cancer cell growth and promoted apoptosis in CRC. A transcriptome analysis combined with immunoprecipitation revealed that hnRNP G-T stabilized 174 mRNAs, including ZDHHC11 mRNA. The cell growth was also suppressed by the transfection of siRNA of ZDHHC11 and the mRNA and the protein expression were decreased by the transfection of siRNA of hnRNP G-T. These results suggested that hnRNP G-T promotes the cell growth of CRC by regulating the mRNA of ZDHHC11. Therefore, hnRNP G-T will be highlighted as an effective therapeutic target with less adverse effects in CRC therapy.
Keywords: ATM; ATR; ZDHHC11; colorectal cancer; hnRNP G-T.
© 2022 The Authors. Cancer Medicine published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no competing interest.
Figures




Similar articles
-
Heterogenous Nuclear Ribonucleoprotein H1 Promotes Colorectal Cancer Progression through the Stabilization of mRNA of Sphingosine-1-Phosphate Lyase 1.Int J Mol Sci. 2020 Jun 25;21(12):4514. doi: 10.3390/ijms21124514. Int J Mol Sci. 2020. PMID: 32630435 Free PMC article.
-
microRNA-26a and -584 inhibit the colorectal cancer progression through inhibition of the binding of hnRNP A1-CDK6 mRNA.Biochem Biophys Res Commun. 2015 Nov 27;467(4):847-52. doi: 10.1016/j.bbrc.2015.10.055. Epub 2015 Oct 19. Biochem Biophys Res Commun. 2015. PMID: 26494299
-
A tumor-specific modulation of heterogeneous ribonucleoprotein A0 promotes excessive mitosis and growth in colorectal cancer cells.Cell Death Dis. 2020 Apr 17;11(4):245. doi: 10.1038/s41419-020-2439-7. Cell Death Dis. 2020. PMID: 32303675 Free PMC article.
-
Heterogeneous nuclear ribonucleoproteins (hnRNPs) in cellular processes: Focus on hnRNP E1's multifunctional regulatory roles.RNA. 2010 Aug;16(8):1449-62. doi: 10.1261/rna.2254110. Epub 2010 Jun 28. RNA. 2010. PMID: 20584894 Free PMC article. Review.
-
The function of the RNA-binding protein hnRNP in cancer metastasis.J Cancer Res Ther. 2013 Nov;9 Suppl:S129-34. doi: 10.4103/0973-1482.122506. J Cancer Res Ther. 2013. PMID: 24516048 Review.
Cited by
-
The up-regulation of SYNCRIP promotes the proliferation and tumorigenesis via DNMT3A/p16 in colorectal cancer.Sci Rep. 2024 Sep 16;14(1):21570. doi: 10.1038/s41598-024-59575-6. Sci Rep. 2024. PMID: 39284825 Free PMC article.
-
Recapitulating the potential contribution of protein S-palmitoylation in cancer.Cancer Metastasis Rev. 2024 Dec 27;44(1):20. doi: 10.1007/s10555-024-10217-3. Cancer Metastasis Rev. 2024. PMID: 39725785 Review.
References
-
- Arnold M, Sierra M.S, Laversanne M, Soerjomataram I, Jemal A, Bray F, et al. Global patterns and trends in colorectal cancer incidence and mortality. Gut. 2017;66:683‐691. - PubMed
-
- Hope NR, Murray GI. The expression profile of RNA‐binding proteins in primary and metastatic colorectal cancer: relationship of heterogeneous nuclear ribonucleoproteins with prognosis. Hum Pathol. 2011;42(3):393‐402. - PubMed
-
- Ushigome M, Ubagai T, Fukuda H, et al. Up‐regulation of hnRNP A1 gene in sporadic human colorectal cancers. Int J Oncol. 2005;26:635‐640. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

