Effects of Unsuppressed Endogenous Insulin on Pharmacokinetics and/or Pharmacodynamics of Study Insulin in the Healthy: A Retrospective Study
- PMID: 35384402
- PMCID: PMC9546084
- DOI: 10.1002/cpdd.1093
Effects of Unsuppressed Endogenous Insulin on Pharmacokinetics and/or Pharmacodynamics of Study Insulin in the Healthy: A Retrospective Study
Abstract
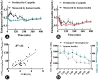
C-peptide, a marker of endogenous insulin, should be consistently inhibited during euglycemic clamping, while an elevated postdosing C-peptide (CPpostdosing ) is not an occasional phenomenon. This was a retrospective study that included 33 men who underwent a manual euglycemic clamp with a subcutaneous injection of insulin aspart (IAsp) aiming to describe the effects of insufficient suppression of endogenous insulin on estimates of the pharmacokinetics and pharmacodynamics of injected insulin. The time profiles of whole blood glucose, human insulin, glucose infusion rate (GIR), and C-peptide were recorded. The subjects were divided into 2 groups at a ratio of 2:1: group A ([CPpostdosing ]max >baseline CP [CPbaseline ]), group B ([CPpostdosing ]max ≤ CPbaseline ). The endogenous insulin was approximately equal to the measured value of human insulin or calculated from the C-peptide. The basal glucose, CPbaseline , basal human insulin, homeostatic model assessment of insulin resistance, IAsp dose, and demographic statistics were all comparable between the 2 groups except the "clamped" glucose. The average clamped glucose was 99.7% (group A) and 94.9% (group B) of baseline. After correction for clamped glucose, GIR area under the concentration-time curve from time 0 to 8 hours was higher in group A (P < .05) under comparable IAsp exposure. Endogenous insulin area under the concentration-time curve from time 0 to 8 hours calculated from C-peptide was different from that measured from human insulin in group A (P < .05), whereas no statistical difference between these measures was observed in group B. Hence, blood glucose should be controlled below the baseline to ensure the inhibition of endogenous insulin. Unsuppressed endogenous insulin may contribute to observed GIR, and the endogenous insulin-corrected pharmacokinetics estimated by C-peptide may be inaccurate with insufficient endogenous insulin suppression.
Keywords: C-peptide; endogenous insulin secretion; euglycemic clamp; insulin pharmacodynamics; insulin pharmacokinetics.
© 2022 The Authors. Clinical Pharmacology in Drug Development published by Wiley Periodicals LLC on behalf of American College of Clinical Pharmacology.
Figures

Similar articles
-
Impact of setting distinct target blood glucose levels on endogenous insulin suppression and pharmacodynamics of insulin preparations.World J Diabetes. 2025 Feb 15;16(2):101779. doi: 10.4239/wjd.v16.i2.101779. World J Diabetes. 2025. PMID: 39959272 Free PMC article.
-
Oscillations of C-peptide in the euglycemic clamp and their effect on the pharmacodynamic assessment of insulin preparations.Fundam Clin Pharmacol. 2021 Aug;35(4):771-780. doi: 10.1111/fcp.12628. Epub 2020 Nov 20. Fundam Clin Pharmacol. 2021. PMID: 33159695
-
Comparison of the pharmacokinetic and pharmacodynamic profiles of biphasic insulin aspart 50 and 30 in patients with type 2 diabetes mellitus: a single-center, randomized, double-blind, two-period, crossover trial in Japan.Clin Ther. 2007 May;29(5):927-934. doi: 10.1016/j.clinthera.2007.05.017. Clin Ther. 2007. PMID: 17697911 Clinical Trial.
-
Clinical pharmacokinetics and pharmacodynamics of insulin aspart.Clin Pharmacokinet. 2001;40(9):641-59. doi: 10.2165/00003088-200140090-00002. Clin Pharmacokinet. 2001. PMID: 11605714 Review.
-
Insulin aspart pharmacokinetics: an assessment of its variability and underlying mechanisms.Eur J Pharm Sci. 2014 Oct 1;62:65-75. doi: 10.1016/j.ejps.2014.05.010. Epub 2014 May 27. Eur J Pharm Sci. 2014. PMID: 24878388 Review.
Cited by
-
How to Achieve Sufficient Endogenous Insulin Suppression in Euglycemic Clamps Assessing the Pharmacokinetics and Pharmacodynamics of Long-Acting Insulin Preparations Employing Healthy Volunteers.Front Pharmacol. 2022 Jul 22;13:899798. doi: 10.3389/fphar.2022.899798. eCollection 2022. Front Pharmacol. 2022. PMID: 35935883 Free PMC article.
-
Impact of setting distinct target blood glucose levels on endogenous insulin suppression and pharmacodynamics of insulin preparations.World J Diabetes. 2025 Feb 15;16(2):101779. doi: 10.4239/wjd.v16.i2.101779. World J Diabetes. 2025. PMID: 39959272 Free PMC article.
-
An in-silico modeling approach to separate exogenous and endogenous plasma insulin appearance, with application to inhaled insulin.Sci Rep. 2024 May 13;14(1):10936. doi: 10.1038/s41598-024-61293-y. Sci Rep. 2024. PMID: 38740832 Free PMC article.
References
-
- Bhatia A, Tawade S, Mastim M, et al. Comparative evaluation of pharmacokinetics and pharmacodynamics of insulin glargine (Glaritus(R) and Lantus(R)) in healthy subjects: a double‐blind, randomized clamp study. Acta Diabetol. 2018;55(5):461‐468. - PubMed
-
- Sinha VP, Choi SL, Soon DK, et al. Single‐dose pharmacokinetics and glucodynamics of the novel, long‐acting basal insulin LY2605541 in healthy subjects. J Clin Pharmacol. 2014;54(7):792‐799. - PubMed
-
- Zhang X, Lam ECQ, Seger ME, et al. LY2963016 Insulin Glargine and Insulin Glargine (Lantus) produce comparable pharmacokinetics and pharmacodynamics at two dose levels. Clin Pharmacol Drug Dev. 2017;6(6):556‐563. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

