Differentiation of Human Induced Pluripotent Stem Cells into Keratinocytes
- PMID: 35384405
- PMCID: PMC9011197
- DOI: 10.1002/cpz1.408
Differentiation of Human Induced Pluripotent Stem Cells into Keratinocytes
Abstract
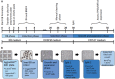
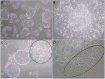
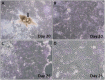
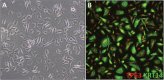
Investigating basic biological mechanisms underlying human diseases relies on the availability of sufficient quantities of patient cells. As most primary somatic cells have a limited lifespan, obtaining sufficient material for biological studies has been a challenge. The development of induced pluripotent stem cell (iPSC) technology has been a game changer, especially in the field of rare genetic disorders. iPSC are essentially immortal, can be stored indefinitely, and can thus be used to generate defined somatic cells in unlimited quantities. Further, the availability of genome editing technologies, such as CRISPR/CAS, has provided us with the opportunity to create "designer" iPSC lines with defined genetic characteristics. A major advancement in biological research stems from the development of methods to direct iPSC differentiation into defined cell types. In this article, we provide the basic protocol for the generation of human iPSC-derived keratinocytes (iPSC-K). These cells have the characteristics of basal epidermal keratinocytes and represent a tool for the investigation of normal epidermal biology, as well as genetic and acquired skin disorders. © 2022 The Authors. Current Protocols published by Wiley Periodicals LLC. Basic Protocol: Directed differentiation of human iPSC into keratinocytes Support Protocol 1: Coating cell culture dishes or plates with Vitronectin XF™ Support Protocol 2: Freezing iPSC Support Protocol 3: Preparing AggreWell™ 400 6-well plates for EB formation Support Protocol 4: Coating cell culture dishes or plates with Collagen IV Support Protocol 5: Immunofluorescence staining of cells.
Keywords: epidermal biology; iPSC; iPSC-derived keratinocytes; induced pluripotent stem cell; keratinocytes; skin diseases.
© 2022 The Authors. Current Protocols published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Similar articles
-
Deriving Keratinocyte Progenitor Cells and Keratinocytes from Human-Induced Pluripotent Stem Cells.Curr Protoc Stem Cell Biol. 2020 Sep;54(1):e119. doi: 10.1002/cpsc.119. Curr Protoc Stem Cell Biol. 2020. PMID: 32744801
-
Differentiation and Subculturing of Renal Proximal Tubular-like Cells Derived from Human iPSC.Curr Protoc. 2023 Aug;3(8):e850. doi: 10.1002/cpz1.850. Curr Protoc. 2023. PMID: 37606532
-
Protocol for the Growth and Maturation of hiPSC-Derived Kidney Organoids using Mechanically Defined Hydrogels.Curr Protoc. 2024 Jul;4(7):e1096. doi: 10.1002/cpz1.1096. Curr Protoc. 2024. PMID: 38984433
-
Genome Editing in Induced Pluripotent Stem Cells using CRISPR/Cas9.Stem Cell Rev Rep. 2018 Jun;14(3):323-336. doi: 10.1007/s12015-018-9811-3. Stem Cell Rev Rep. 2018. PMID: 29623532 Review.
-
Generation of defined neural populations from pluripotent stem cells.Philos Trans R Soc Lond B Biol Sci. 2018 Jul 5;373(1750):20170214. doi: 10.1098/rstb.2017.0214. Philos Trans R Soc Lond B Biol Sci. 2018. PMID: 29786550 Free PMC article. Review.
Cited by
-
Effects of TP63 mutations on keratinocyte adhesion and migration.Exp Dermatol. 2023 Sep;32(9):1575-1581. doi: 10.1111/exd.14885. Epub 2023 Jul 11. Exp Dermatol. 2023. PMID: 37432020 Free PMC article.
-
A live-cell image-based machine learning strategy for reducing variability in PSC differentiation systems.Cell Discov. 2023 Jun 6;9(1):53. doi: 10.1038/s41421-023-00543-1. Cell Discov. 2023. PMID: 37280224 Free PMC article.
-
Twin Prime Editing Mediated Exon Skipping/Reinsertion for Restored Collagen VII Expression in Recessive Dystrophic Epidermolysis Bullosa.J Invest Dermatol. 2024 Dec;144(12):2764-2777.e9. doi: 10.1016/j.jid.2024.04.013. Epub 2024 May 17. J Invest Dermatol. 2024. PMID: 38763174
-
Human-Induced Pluripotent Stem Cell‒Derived Keratinocytes, as Therapeutic Option in Vitiligo.Methods Mol Biol. 2024;2849:185-202. doi: 10.1007/7651_2023_510. Methods Mol Biol. 2024. PMID: 38189899
-
Dynamic 3D Combinatorial Generation of hPSC-Derived Neuromesodermal Organoids With Diverse Regional and Cellular Identities.Curr Protoc. 2022 Oct;2(10):e568. doi: 10.1002/cpz1.568. Curr Protoc. 2022. PMID: 36264199 Free PMC article.
References
-
- Dinella, J. D. , Chen, J. , Webb, S. , Siegfried, E. , Bree, A. F. , Lakshmanachetty, S. , … Koch, P. J. (2018). A human stem cell‐based system to study the role of TP63 mutations in ectodermal dysplasias. Journal of Investigative Dermatology, 138(7), 1662–1665. doi: 10.1016/j.jid.2018.02.016. - DOI - PMC - PubMed
-
- Green, H. , Rheinwald, J. G. , & Sun, T. T. (1977). Properties of an epithelial cell type in culture: The epidermal keratinocyte and its dependence on products of the fibroblast. Progress in Clinical and Biological Research, 17, 493–500. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

