Breakthrough Infections Following mRNA SARS-CoV-2 Vaccination in Kidney Transplant Recipients
- PMID: 35384924
- PMCID: PMC9213063
- DOI: 10.1097/TP.0000000000004119
Breakthrough Infections Following mRNA SARS-CoV-2 Vaccination in Kidney Transplant Recipients
Abstract
Background: The clinical effectiveness of coronavirus disease 2019 (COVID-19) vaccination in kidney transplant (KT) recipients is lower than in the general population.
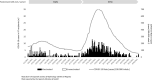
Methods: From April to October 2021, 481 KT recipients with COVID-19, included in the Spanish Society of Nephrology COVID-19 Registry, were analyzed. Data regarding vaccination status and vaccine type were collected, and outcomes of unvaccinated or partially vaccinated patients (n = 130) were compared with fully vaccinated patients (n = 351).
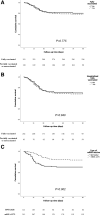
Results: Clinical picture was similar and survival analysis showed no differences between groups: 21.7% of fully vaccinated patients and 20.8% of unvaccinated or partially vaccinated died (P = 0.776). In multivariable analysis, age and pneumonia were independent risk factors for death, whereas vaccination status was not related to mortality. These results remained similar when we excluded patients with partial vaccination, as well as when we analyzed exclusively hospitalized patients. Patients vaccinated with mRNA-1273 (n = 213) showed a significantly lower mortality than those who received the BNT162b2 vaccine (n = 121) (hazard ratio: 0.52; 95% confidence interval, 0.31-0.85; P = 0.010).
Conclusions: COVID-19 severity in KT patients has remained high and has not improved despite receiving 2 doses of the mRNA vaccine. The mRNA-1273 vaccine shows higher clinical effectiveness than BNT162b2 in KT recipients with breakthrough infections. Confirmation of these data will require further research taking into account the new variants and the administration of successive vaccine doses.
Copyright © 2022 Wolters Kluwer Health, Inc. All rights reserved.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- World Health Organization. WHO Coronavirus (COVID-19) Dashborad. 2021. Available at https://covid19.who.int/. Accessed November 25, 2021.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

