Efficacy of Liposomal Bupivacaine and Bupivacaine Hydrochloride vs Bupivacaine Hydrochloride Alone as a Periarticular Anesthetic for Patients Undergoing Knee Replacement: A Randomized Clinical Trial
- PMID: 35385072
- PMCID: PMC8988023
- DOI: 10.1001/jamasurg.2022.0713
Efficacy of Liposomal Bupivacaine and Bupivacaine Hydrochloride vs Bupivacaine Hydrochloride Alone as a Periarticular Anesthetic for Patients Undergoing Knee Replacement: A Randomized Clinical Trial
Abstract
Importance: More than half of patients who undergo knee replacement surgery report substantial acute postoperative pain.
Objective: To evaluate the efficacy and cost-effectiveness of periarticular liposomal bupivacaine for recovery and pain management after knee replacement.
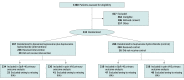
Design, setting, and participants: This multicenter, patient-blinded, pragmatic, randomized clinical superiority trial involved 533 participants at 11 institutions within the National Health Service in England. Adults undergoing primary unilateral knee replacement for symptomatic end-stage osteoarthritis were enrolled between March 29, 2018, and February 29, 2020, and followed up for 1 year after surgery. Follow-up was completed March 1, 2021. A per-protocol analysis for each coprimary outcome was performed in addition to the main intention-to-treat analysis.
Interventions: Two hundred sixty-six milligrams of liposomal bupivacaine admixed with 100 mg of bupivacaine hydrochloride compared with 100 mg of bupivacaine hydrochloride alone (control) administered by periarticular injection at the time of surgery.
Main outcome and measures: The coprimary outcomes were Quality of Recovery 40 (QoR-40) score at 72 hours and pain visual analog scale (VAS) score area under the curve (AUC) from 6 to 72 hours. Secondary outcomes included QoR-40 and mean pain VAS at days 0 (evening of surgery), 1, 2, and 3; cumulative opioid consumption for 72 hours; functional outcomes and quality of life at 6 weeks, 6 months, and 1 year; and cost-effectiveness for 1 year. Adverse events and serious adverse events up to 12 months after randomization were also assessed.
Results: Among the 533 participants included in the analysis, the mean (SD) age was 69.0 (9.7) years; 287 patients were women (53.8%) and 246 were men (46.2%). Baseline characteristics were balanced between study groups. There was no difference between the liposomal bupivacaine and control groups in QoR-40 score at 72 hours (adjusted mean difference, 0.54 [97.5% CI, -2.05 to 3.13]; P = .64) or the pain VAS score AUC at 6 to 72 hours (-21.5 [97.5% CI, -46.8 to 3.8]; P = .06). Analyses of pain VAS and QoR-40 scores demonstrated only 1 statistically significant difference, with the liposomal bupivacaine arm having lower pain scores the evening of surgery (adjusted difference -0.54 [97.5% CI, -1.07 to -0.02]; P = .02). No difference in cumulative opioid consumption and functional outcomes was detected. Liposomal bupivacaine was not cost-effective compared with the control treatment. No difference in adverse or serious adverse events was found between the liposomal bupivacaine and control groups.
Conclusions and relevance: This study found no difference in postoperative recovery or pain associated with the use of periarticular liposomal bupivacaine compared with bupivacaine hydrochloride alone in patients who underwent knee replacement surgery.
Trial registration: isrctn.com Identifier: ISRCTN54191675.
Conflict of interest statement
Figures


Comment in
-
Liposomal Bupivacaine-A Boon for Opioid-Sparing Surgery?-Reply.JAMA Surg. 2022 Oct 1;157(10):968-969. doi: 10.1001/jamasurg.2022.2814. JAMA Surg. 2022. PMID: 35857299 No abstract available.
-
Liposomal Bupivacaine-A Boon for Opioid-Sparing Surgery?JAMA Surg. 2022 Oct 1;157(10):968. doi: 10.1001/jamasurg.2022.2811. JAMA Surg. 2022. PMID: 35857302 No abstract available.
References
-
- Strickland LH, Kelly L, Hamilton TW, Murray DW, Pandit HG, Jenkinson C. Early recovery following lower limb arthroplasty: qualitative interviews with patients undergoing elective hip and knee replacement surgery. Initial phase in the development of a patient-reported outcome measure. J Clin Nurs. 2018;27(13-14):2598-2608. doi: 10.1111/jocn.14086 - DOI - PubMed

