Fluvoxamine for Outpatient Management of COVID-19 to Prevent Hospitalization: A Systematic Review and Meta-analysis
- PMID: 35385087
- PMCID: PMC8987902
- DOI: 10.1001/jamanetworkopen.2022.6269
Fluvoxamine for Outpatient Management of COVID-19 to Prevent Hospitalization: A Systematic Review and Meta-analysis
Abstract
Importance: Widely available and affordable options for the outpatient management of COVID-19 are needed, particularly for therapies that prevent hospitalization.
Objective: To perform a meta-analysis of the available randomized clinical trial evidence for fluvoxamine in the outpatient management of COVID-19.
Data sources: World Health Organization International Clinical Trials Registry Platform and ClinicalTrials.gov.
Study selection: Studies with completed outpatient trials with available results that compared fluvoxamine with placebo were included.
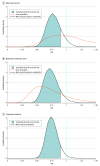
Data extraction and synthesis: The PRISMA 2020 guidelines were followed and study details in terms of inclusion criteria, trial demographics, and the prespecified outcome of all-cause hospitalization were extracted. Risk of bias was assessed by the Cochrane Risk of Bias 2 tool and a bayesian random effects meta-analysis with different estimates of prior probability was conducted: a weakly neutral prior (50% chance of efficacy with 95% CI for risk ratio [RR] between 0.5 and 2.0) and a moderately optimistic prior (85% chance of efficacy). A frequentist random-effects meta-analysis was conducted as a senstivity analysis, and the results were contextualized by estimating the probability of any association (RR ≤ 1) and moderate association (RR ≤ 0.9) with reduced hospitalization.
Main outcomes and measures: All-cause hospitalization.
Results: This systematic review and meta-analysis of 3 randomized clinical trials and included 2196 participants. The RRs for hospitalization were 0.78 (95% CI, 0.58-1.08) for the bayesian weakly neutral prior, 0.73 (95% CI, 0.53-1.01) for the bayesian moderately optimistic prior, and 0.75 (95% CI, 0.58-0.97) for the frequentist analysis. Depending on the scenario, the probability of any association with reduced hospitalization ranged from 94.1% to 98.6%, and the probability of moderate association ranged from 81.6% to 91.8%.
Conclusions and relevance: In this systematic review and meta-analysis of data from 3 trials, under a variety of assumptions, fluvoxamine showed a high probability of being associated with reduced hospitalization in outpatients with COVID-19. Ongoing randomized trials are important to evaluate alternative doses, explore the effectiveness in vaccinated patients, and provide further refinement to these estimates. Meanwhile, fluvoxamine could be recommended as a management option, particularly in resource-limited settings or for individuals without access to SARS-CoV-2 monoclonal antibody therapy or direct antivirals.
Conflict of interest statement
Figures
Similar articles
-
Fluvoxamine in Nonhospitalized Patients With Acute COVID-19 Infection and the Lack of Efficacy in Reducing Rates of Hospitalization, Mechanical Ventilation, and Mortality in Placebo-Controlled Trials: A Systematic Review and Meta-Analysis.Am J Ther. 2022 May-Jun 01;29(3):e298-e304. doi: 10.1097/MJT.0000000000001496. Epub 2022 Mar 31. Am J Ther. 2022. PMID: 35383578
-
The efficacy and safety of fluvoxamine in patients with COVID-19: A systematic review and meta-analysis from randomized controlled trials.PLoS One. 2024 May 16;19(5):e0300512. doi: 10.1371/journal.pone.0300512. eCollection 2024. PLoS One. 2024. PMID: 38753761 Free PMC article.
-
Efficacy and Safety of Fluvoxamine as Outpatient Treatment for Patients With Covid-19: A Systematic Review and Meta-analysis of Clinical Trials.Ann Pharmacother. 2023 Dec;57(12):1389-1397. doi: 10.1177/10600280231162243. Epub 2023 Mar 31. Ann Pharmacother. 2023. PMID: 37002592 Free PMC article.
-
Effect of Fluvoxamine vs Placebo on Time to Sustained Recovery in Outpatients With Mild to Moderate COVID-19: A Randomized Clinical Trial.JAMA. 2023 Jan 24;329(4):296-305. doi: 10.1001/jama.2022.24100. JAMA. 2023. PMID: 36633838 Free PMC article.
-
Higher-Dose Fluvoxamine and Time to Sustained Recovery in Outpatients With COVID-19: The ACTIV-6 Randomized Clinical Trial.JAMA. 2023 Dec 26;330(24):2354-2363. doi: 10.1001/jama.2023.23363. JAMA. 2023. PMID: 37976072 Free PMC article.
Cited by
-
Outpatient Treatment of Confirmed COVID-19: A Living, Rapid Review for the American College of Physicians.Ann Intern Med. 2023 Jan;176(1):92-104. doi: 10.7326/M22-2202. Epub 2022 Nov 29. Ann Intern Med. 2023. Update in: Ann Intern Med. 2023 Oct;176(10):1377-1385. doi: 10.7326/M23-1626. PMID: 36442056 Free PMC article. Updated. Review.
-
A Newly Engineered A549 Cell Line Expressing ACE2 and TMPRSS2 Is Highly Permissive to SARS-CoV-2, Including the Delta and Omicron Variants.Viruses. 2022 Jun 23;14(7):1369. doi: 10.3390/v14071369. Viruses. 2022. PMID: 35891350 Free PMC article.
-
Efficacy and safety of selective serotonin reuptake inhibitors in COVID-19 management: a systematic review and meta-analysis.Clin Microbiol Infect. 2023 May;29(5):578-586. doi: 10.1016/j.cmi.2023.01.010. Epub 2023 Jan 16. Clin Microbiol Infect. 2023. PMID: 36657488 Free PMC article.
-
Molecular Bases of Serotonin Reuptake Inhibitor Antidepressant-Attributed Effects in COVID-19: A New Insight on the Role of Bradykinins.J Pers Med. 2022 Sep 11;12(9):1487. doi: 10.3390/jpm12091487. J Pers Med. 2022. PMID: 36143272 Free PMC article. Review.
-
COVID-19 Molecular Pathophysiology: Acetylation of Repurposing Drugs.Int J Mol Sci. 2022 Oct 31;23(21):13260. doi: 10.3390/ijms232113260. Int J Mol Sci. 2022. PMID: 36362045 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous