Twin-free, directly synthesized MFI nanosheets with improved thickness uniformity and their use in membrane fabrication
- PMID: 35385314
- PMCID: PMC8986103
- DOI: 10.1126/sciadv.abm8162
Twin-free, directly synthesized MFI nanosheets with improved thickness uniformity and their use in membrane fabrication
Abstract
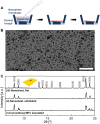
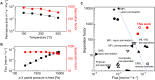
Zeolite nanosheets can be used for the fabrication of low-defect-density, thin, and oriented zeolite separation membranes. However, methods for manipulating their morphology are limited, hindering progress toward improved performance. We report the direct synthesis (i.e., without using exfoliation, etching, or other top-down processing) of thin, flat MFI nanosheets and demonstrate their use as high-performance membranes for xylene isomer separations. Our MFI nanosheets were synthesized using nanosheet fragments as seeds instead of the previously used MFI nanoparticles. The obtained MFI nanosheets exhibit improved thickness uniformity and are free of rotational and MEL intergrowths as shown by transmission electron microscopy (TEM) imaging. The nanosheets can form well-packed nanosheet coatings. Upon gel-free secondary growth, the obtained zeolite MFI membranes show high separation performance for xylene isomers at elevated temperature (e.g., p-xylene flux up to 1.5 × 10-3 mol m-2 s-1 and p-/o-xylene separation factor of ~600 at 250°C).
Figures




References
-
- Kondo M., Komori M., Kita H., Okamoto K., Tubular-type pervaporation module with zeolite NaA membrane. J. Membr. Sci. 133, 133–141 (1997).
-
- Carreon M. A., Li S., Falconer J. L., Noble R. D., Alumina-supported SAPO-34 membranes for CO2/CH4 separation. J. Am. Chem. Soc. 130, 5412–5413 (2008). - PubMed
-
- Okazaki J., Hasegawa H., Chikamatsu N., Yajima K., Shimizu K., Niino M., DDR-type zeolite membrane: A novel CO2 separation technology for enhanced oil recovery. Sep. Purif. Technol. 218, 200–205 (2019).
-
- Rangnekar N., Mittal N., Elyassi B., Caro J., Tsapatsis M., Zeolite membranes –A review and comparison with MOFs. Chem. Soc. Rev. 44, 7128–7154 (2015). - PubMed
-
- Sawamura K., Furuhata T., Sekine Y., Kikuchi E., Subramanian B., Matsukata M., Zeolite membrane for dehydration of isopropylalcohol–water mixture by vapor permeation. ACS Appl. Mater. Interfaces 7, 13728–13730 (2015). - PubMed
LinkOut - more resources
Full Text Sources

