Controlled assembly of retinal cells on fractal and Euclidean electrodes
- PMID: 35385490
- PMCID: PMC8985931
- DOI: 10.1371/journal.pone.0265685
Controlled assembly of retinal cells on fractal and Euclidean electrodes
Abstract
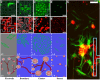
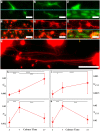
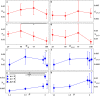
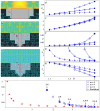
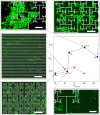
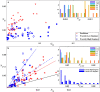
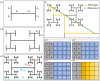
Controlled assembly of retinal cells on artificial surfaces is important for fundamental cell research and medical applications. We investigate fractal electrodes with branches of vertically-aligned carbon nanotubes and silicon dioxide gaps between the branches that form repeating patterns spanning from micro- to milli-meters, along with single-scaled Euclidean electrodes. Fluorescence and electron microscopy show neurons adhere in large numbers to branches while glial cells cover the gaps. This ensures neurons will be close to the electrodes' stimulating electric fields in applications. Furthermore, glia won't hinder neuron-branch interactions but will be sufficiently close for neurons to benefit from the glia's life-supporting functions. This cell 'herding' is adjusted using the fractal electrode's dimension and number of repeating levels. We explain how this tuning facilitates substantial glial coverage in the gaps which fuels neural networks with small-world structural characteristics. The large branch-gap interface then allows these networks to connect to the neuron-rich branches.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Miscellaneous

