Structural Diversity in Multicomponent Nanocrystal Superlattices Comprising Lead Halide Perovskite Nanocubes
- PMID: 35385663
- PMCID: PMC9134504
- DOI: 10.1021/acsnano.1c10702
Structural Diversity in Multicomponent Nanocrystal Superlattices Comprising Lead Halide Perovskite Nanocubes
Abstract
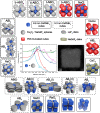
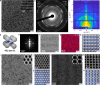
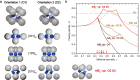
Nanocrystal (NC) self-assembly is a versatile platform for materials engineering at the mesoscale. The NC shape anisotropy leads to structures not observed with spherical NCs. This work presents a broad structural diversity in multicomponent, long-range ordered superlattices (SLs) comprising highly luminescent cubic CsPbBr3 NCs (and FAPbBr3 NCs) coassembled with the spherical, truncated cuboid, and disk-shaped NC building blocks. CsPbBr3 nanocubes combined with Fe3O4 or NaGdF4 spheres and truncated cuboid PbS NCs form binary SLs of six structure types with high packing density; namely, AB2, quasi-ternary ABO3, and ABO6 types as well as previously known NaCl, AlB2, and CuAu types. In these structures, nanocubes preserve orientational coherence. Combining nanocubes with large and thick NaGdF4 nanodisks results in the orthorhombic SL resembling CaC2 structure with pairs of CsPbBr3 NCs on one lattice site. Also, we implement two substrate-free methods of SL formation. Oil-in-oil templated assembly results in the formation of binary supraparticles. Self-assembly at the liquid-air interface from the drying solution cast over the glyceryl triacetate as subphase yields extended thin films of SLs. Collective electronic states arise at low temperatures from the dense, periodic packing of NCs, observed as sharp red-shifted bands at 6 K in the photoluminescence and absorption spectra and persisting up to 200 K.
Keywords: binary superlattice; collective properties; colloidal nanocrystals; electron microscopy; lead halide perovskites; nanocrystal shape; self-assembly.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
















Similar articles
-
All-Perovskite Multicomponent Nanocrystal Superlattices.ACS Nano. 2024 Mar 19;18(11):8423-8436. doi: 10.1021/acsnano.3c13062. Epub 2024 Mar 6. ACS Nano. 2024. PMID: 38446635 Free PMC article.
-
Shape-Directed Co-Assembly of Lead Halide Perovskite Nanocubes with Dielectric Nanodisks into Binary Nanocrystal Superlattices.ACS Nano. 2021 Oct 26;15(10):16488-16500. doi: 10.1021/acsnano.1c06047. Epub 2021 Sep 22. ACS Nano. 2021. PMID: 34549582 Free PMC article.
-
Perovskite-type superlattices from lead halide perovskite nanocubes.Nature. 2021 May;593(7860):535-542. doi: 10.1038/s41586-021-03492-5. Epub 2021 May 26. Nature. 2021. PMID: 34040208
-
Colloidal Self-Assembly of Inorganic Nanocrystals into Superlattice Thin-Films and Multiscale Nanostructures.Nanomaterials (Basel). 2019 Sep 1;9(9):1243. doi: 10.3390/nano9091243. Nanomaterials (Basel). 2019. PMID: 31480547 Free PMC article. Review.
-
Perovskite nanocrystal superlattices: self-assembly, collective behavior, and applications.Chem Commun (Camb). 2023 May 2;59(36):5365-5374. doi: 10.1039/d2cc06534g. Chem Commun (Camb). 2023. PMID: 37070699 Review.
Cited by
-
Emergent Properties from Three-Dimensional Assemblies of (Nano)particles in Confined Spaces.Cryst Growth Des. 2024 Apr 17;24(14):6060-6080. doi: 10.1021/acs.cgd.4c00260. eCollection 2024 Jul 17. Cryst Growth Des. 2024. PMID: 39044735 Free PMC article. Review.
-
Collective Diffraction Effects in Perovskite Nanocrystal Superlattices.Acc Chem Res. 2023 Jan 3;56(1):66-76. doi: 10.1021/acs.accounts.2c00613. Epub 2022 Dec 19. Acc Chem Res. 2023. PMID: 36534898 Free PMC article.
-
Long live(d) CsPbBr3 superlattices: colloidal atomic layer deposition for structural stability.Chem Sci. 2024 Feb 19;15(12):4510-4518. doi: 10.1039/d3sc06662b. eCollection 2024 Mar 20. Chem Sci. 2024. PMID: 38516096 Free PMC article.
-
All-Perovskite Multicomponent Nanocrystal Superlattices.ACS Nano. 2024 Mar 19;18(11):8423-8436. doi: 10.1021/acsnano.3c13062. Epub 2024 Mar 6. ACS Nano. 2024. PMID: 38446635 Free PMC article.
-
Ensemble hot-spots in 3D supercrystals of plasmonic octahedral nanoparticles in tip-to-tip configured superlattices.Nat Commun. 2025 Mar 20;16(1):2762. doi: 10.1038/s41467-025-58029-5. Nat Commun. 2025. PMID: 40113774 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Miscellaneous

