ALS-associated KIF5A mutations abolish autoinhibition resulting in a toxic gain of function
- PMID: 35385738
- PMCID: PMC9134378
- DOI: 10.1016/j.celrep.2022.110598
ALS-associated KIF5A mutations abolish autoinhibition resulting in a toxic gain of function
Abstract
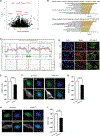
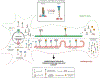
Understanding the pathogenic mechanisms of disease mutations is critical to advancing treatments. ALS-associated mutations in the gene encoding the microtubule motor KIF5A result in skipping of exon 27 (KIF5AΔExon27) and the encoding of a protein with a novel 39 amino acid residue C-terminal sequence. Here, we report that expression of ALS-linked mutant KIF5A results in dysregulated motor activity, cellular mislocalization, altered axonal transport, and decreased neuronal survival. Single-molecule analysis revealed that the altered C terminus of mutant KIF5A results in a constitutively active state. Furthermore, mutant KIF5A possesses altered protein and RNA interactions and its expression results in altered gene expression/splicing. Taken together, our data support the hypothesis that causative ALS mutations result in a toxic gain of function in the intracellular motor KIF5A that disrupts intracellular trafficking and neuronal homeostasis.
Keywords: ALS; CP: Neuroscience; KIF5A; amyotrophic lateral sclerosis; autoinhibition; axonal transport; kinesin; neurodegenerative disease; neuronal survival.
Copyright © 2022 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests J.E.L. is a member of the scientific advisory board for Cerevel Therapeutics, a consultant for ACI Clinical LLC sponsored by Biogen, Inc. and Ionis Pharmaceuticals, Inc. J.E.L. is also a consultant for Perkins Coie LLP and may provide expert testimony.
Figures




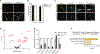
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

