A Novel Faster-Acting, Dry Powder-Based, Naloxone Intranasal Formulation for Opioid Overdose
- PMID: 35386013
- PMCID: PMC9160115
- DOI: 10.1007/s11095-022-03247-5
A Novel Faster-Acting, Dry Powder-Based, Naloxone Intranasal Formulation for Opioid Overdose
Abstract
Objective: To examine the pharmacokinetics and safety of FMXIN001, a new intranasal powder-based naloxone formulation, in comparison to Narcan® nasal liquid spray.
Methods: FMXIN001, was developed by blending drug microspheres with larger lactose monohydrate particles, that serve as diluent and carrier, as well as a disaggregating agent. Scanning electron microscopy and X-ray were used to characterize the formulation and in vitro deposition was investigated using a nasal cast. We compared the pharmacokinetics and safety of FMXIN001 versus Narcan® in two clinical trials: a pilot study with 14 healthy adults and a pivotal trial in 42 healthy adults (NCT04713709). The studies were open-label, single-dose, randomized, two-period, two-treatment, two-sequence crossover studies to assess the pharmacokinetics and safety of FMXIN001 versus Narcan® nasal spray.
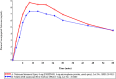
Results: FMXIN001 comprises naloxone microspheres (5-30 μM) and lactose particles (40-240 μM). Upon in vitro testing, naloxone deposits mainly to the middle turbinates region and the upper part of the nasal cavity of a nasal cast. In human subjects, FMXIN001 produced significantly higher exposure at the initial time points of 4, 10, and 30 min, post-administration, compared to Narcan®. Both treatments were safe and well tolerated. FMXIN001, powder-based spray, results in similar overall exposure to Narcan®, but with more rapid absorption in the first 30 min.
Conclusions: FMXIN001 is expected to have a shorter onset of action for a more effective therapeutic intervention to manage opioid overdose. Rapid administration of naloxone in cases of opioid overdose is imperative, given the alarming increase in mortality rates.
Keywords: bioavailability; dry powder; lactose; naloxone microspheres; nasal spray.
© 2022. The Author(s).
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

