Dual-functional porous and cisplatin-loaded polymethylmethacrylate cement for reconstruction of load-bearing bone defect kills bone tumor cells
- PMID: 35386344
- PMCID: PMC8941180
- DOI: 10.1016/j.bioactmat.2021.12.023
Dual-functional porous and cisplatin-loaded polymethylmethacrylate cement for reconstruction of load-bearing bone defect kills bone tumor cells
Abstract
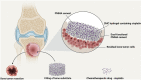
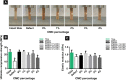
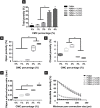
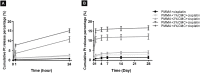
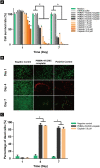
Malignant bone tumors are usually treated by resection of tumor tissue followed by filling of the bone defect with bone graft substitutes. Polymethylmethacrylate (PMMA) cement is the most commonly used bone substitute in clinical orthopedics in view of its reliability. However, the dense nature of PMMA renders this biomaterial unsuitable for local delivery of chemotherapeutic drugs to limit the recurrence of bone tumors. Here, we introduce porosity into PMMA cement by adding carboxymethylcellulose (CMC) to facilitate such local delivery of chemotherapeutic drugs, while retaining sufficient mechanical properties for bone reconstruction in load-bearing sites. Our results show that the mechanical strength of PMMA-based cements gradually decreases with increasing CMC content. Upon incorporation of ≥3% CMC, the PMMA-based cements released up to 18% of the loaded cisplatin, in contrast to cements containing lower amounts of CMC which only released less than 2% of the cisplatin over 28 days. This release of cisplatin efficiently killed osteosarcoma cells in vitro and the fraction of dead cells increased to 91.3% at day 7, which confirms the retained chemotherapeutic activity of released cisplatin from these PMMA-based cements. Additionally, tibias filled with PMMA-based cements containing up to 3% of CMC exhibit comparable compressive strengths as compared to intact tibias. In conclusion, we demonstrate that PMMA cements can be rendered therapeutically active by introducing porosity using CMC to allow for release of cisplatin without compromising mechanical properties beyond critical levels. As such, these data suggest that our dual-functional PMMA-based cements represent a viable treatment option for filling bone defects after bone tumor resection in load-bearing sites.
Keywords: Bone tumor treatment; Chemotherapeutic drug; Ex vivo biomechanical assessment; Local drug delivery; Porous polymethylmethacrylate cement.
© 2022 The Authors.
Figures




References
-
- Gartrell B.A., Saad F. Managing bone metastases and reducing skeletal related events in prostate cancer. Nat. Rev. Clin. Oncol. 2014;11(6):335–345. - PubMed
-
- Link M.P., Goorin A.M., Horowitz M., Meyer W.H., Belasco J., Baker A., et al. Adjuvant chemotherapy of high-grade osteosarcoma of the extremity. Updated results of the Multi-Institutional Osteosarcoma Study. Clin. Orthop. Relat. Res. 1991;(270):8–14. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

