Rational design of biodegradable thermoplastic polyurethanes for tissue repair
- PMID: 35386346
- PMCID: PMC8940769
- DOI: 10.1016/j.bioactmat.2021.11.029
Rational design of biodegradable thermoplastic polyurethanes for tissue repair
Abstract
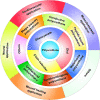
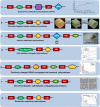
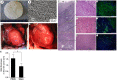
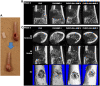
As a type of elastomeric polymers, non-degradable polyurethanes (PUs) have a long history of being used in clinics, whereas biodegradable PUs have been developed in recent decades, primarily for tissue repair and regeneration. Biodegradable thermoplastic (linear) PUs are soft and elastic polymeric biomaterials with high mechanical strength, which mimics the mechanical properties of soft and elastic tissues. Therefore, biodegradable thermoplastic polyurethanes are promising scaffolding materials for soft and elastic tissue repair and regeneration. Generally, PUs are synthesized by linking three types of changeable blocks: diisocyanates, diols, and chain extenders. Alternating the combination of these three blocks can finely tailor the physio-chemical properties and generate new functional PUs. These PUs have excellent processing flexibilities and can be fabricated into three-dimensional (3D) constructs using conventional and/or advanced technologies, which is a great advantage compared with cross-linked thermoset elastomers. Additionally, they can be combined with biomolecules to incorporate desired bioactivities to broaden their biomedical applications. In this review, we comprehensively summarized the synthesis, structures, and properties of biodegradable thermoplastic PUs, and introduced their multiple applications in tissue repair and regeneration. A whole picture of their design and applications along with discussions and perspectives of future directions would provide theoretical and technical supports to inspire new PU development and novel applications.
Keywords: Biodegradable polyurethane; Elastic; Synthesis; Thermoplastic; Tissue repair.
© 2021 The Authors.
Figures





References
-
- Burdick J.A., Mauck R.L. 1 st ed. Springer-Verlag/Wien; 2011. Biomaterials for Tissue Engineering Applications: a Review of the Past and Future Trends.
-
- O'Brien F.J. Biomaterials & scaffolds for tissue engineering. Mater. Today. 2011;14(3):88–95.
-
- Yang S., Leong K.F., Du Z., Chua C.K. The design of scaffolds for use in tissue engineering. Part I. Traditional factors. Tissue Eng. 2001;7(6):679–689. - PubMed
-
- Cooper S.L., Guan J. Advances in Polyurethane Biomaterials. Elsevier; 2016.
-
- Lamba N.M., Woodhouse K.A., Cooper S.L. CRC press; 1998. Polyurethanes in Biomedical Applications.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

