Age-dependent phenotypes of ovarian endometriomas
- PMID: 35386381
- PMCID: PMC8967305
- DOI: 10.1002/rmb2.12438
Age-dependent phenotypes of ovarian endometriomas
Abstract
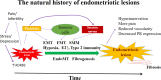
Purpose: To analyze the characteristics of the ovarian endometrioma (OE) across the life span of a woman. In the past, the OE has traditionally been viewed as a single, monolithic disease. Today, there are emerging data indicating that OE phenotypes differ according to the age of the woman.
Method: A narrative review of original articles on OE indexed by PubMed.
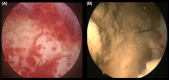
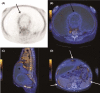
Results: When appearing in infancy and early adolescence, OE may be the consequence of endometrial cells retrogradely shed with neonatal uterine bleeding. The post-menarcheal variant, manifesting itself during full adolescence, is singularly frequent in the presence of vaginal or uterine outflow obstructive anomalies. The typical and most frequent adult phenotype is characterized by increasing fibrosis and a tendency to progress; its mere presence exerts a detrimental effect on the surrounding healthy ovarian tissue. In postmenopause, an old lesion may be reactivated in the presence of exogenous or endogenous estrogens, or even be produced ex novo; rarely, it can spread to a variety of organs and structures and even degenerate causing malignancies.
Conclusions: Given the existence of these variants, it is important to agree on management guidelines that take into consideration these different phenotypes.
Keywords: adolescence; adulthood; neonatal uterine bleeding; ovarian endometrioma; postmenopause; premenarche.
© 2022 The Authors. Reproductive Medicine and Biology published by John Wiley & Sons Australia, Ltd on behalf of Japan Society for Reproductive Medicine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Jenkins S, Olive DL, Haney AF. Endometriosis: pathogenetic implications of the anatomic distribution. Obstet Gynecol. 1986;67(3):335‐338. - PubMed
-
- Gylfason JT, Kristjansson KA, Sverrisdottir G, Jonsdottir K, Rafnsson V, Geirsson RT. Pelvic endometriosis diagnosed in an entire nation over 20 years. Am J Epidemiol. 2010;172(3):237‐243. - PubMed
-
- Somigliana E. Ovarian reserve, endometriomas, and surgery: research must go on. Fertil Steril. 2018;110(5):856‐857. - PubMed
-
- Blair BW. Endometrioma and endometriomyoma of the ovary. J Obstetr Gynaecol Brit Emp. 1922;29:443‐446.
-
- Brosens I, Puttemans P, Benagiano G. Endometriosis: a life cycle approach? Am J Obstet Gynecol. 2013;209(4):307‐316. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous
