COVID-19 vaccine-associated organizing pneumonia
- PMID: 35386579
- PMCID: PMC8965045
- DOI: 10.1002/rcr2.944
COVID-19 vaccine-associated organizing pneumonia
Abstract
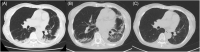
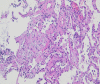
We report the first case of organizing pneumonia (OP) associated with a new coronavirus disease (COVID-19) vaccination. A 78-year-old woman developed cough and dyspnoea 10 days after receiving BNT162b2. Chest computed tomography (CT) revealed consolidation in the bilateral lower lobes of the lungs. Although antibiotic treatment did not improve her symptoms, she received a second vaccination as scheduled. She was referred to our hospital because of worsening dyspnoea on day 9 after the second vaccination, with reversed halo signs in the bilateral lower pulmonary lobes and new consolidation in the left lingual region on chest CT on day 15. She was diagnosed with OP based on bronchoalveolar lavage and transbronchial lung biopsy findings. Treatment with oral prednisolone 0.5 mg/kg/day immediately improved the symptoms and chest imaging findings. In the absence of other triggering factors, we considered this case as being COVID-19 vaccine-associated following the first and second vaccinations.
Keywords: COVID‐19; adverse events; interstitial lung disease; organizing pneumonia; vaccine.
© 2022 The Authors. Respirology Case Reports published by John Wiley & Sons Australia, Ltd on behalf of The Asian Pacific Society of Respirology.
Conflict of interest statement
None declared.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

