Role of Th17 Cytokines in Airway Remodeling in Asthma and Therapy Perspectives
- PMID: 35386663
- PMCID: PMC8974749
- DOI: 10.3389/falgy.2022.806391
Role of Th17 Cytokines in Airway Remodeling in Asthma and Therapy Perspectives
Abstract
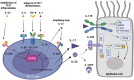
Airway remodeling is a frequent pathological feature of severe asthma leading to permanent airway obstruction in up to 50% of cases and to respiratory disability. Although structural changes related to airway remodeling are well-characterized, immunological processes triggering and maintaining this phenomenon are still poorly understood. As a consequence, no biotherapy targeting cytokines are currently efficient to treat airway remodeling and only bronchial thermoplasty may have an effect on bronchial nerves and smooth muscles with uncertain clinical relevance. Th17 cytokines, including interleukin (IL)-17 and IL-22, play a role in neutrophilic inflammation in severe asthma and may be involved in airway remodeling. Indeed, IL-17 is increased in sputum from severe asthmatic patients, induces the expression of "profibrotic" cytokines by epithelial, endothelial cells and fibroblasts, and provokes human airway smooth muscle cell migration in in vitro studies. IL-22 is also increased in asthmatic samples, promotes myofibroblast differentiation, epithelial-mesenchymal transition and proliferation and migration of smooth muscle cells in vitro. Accordingly, we also found high levels of IL-17 and IL-22 in a mouse model of dog-allergen induced asthma characterized by a strong airway remodeling. Clinical trials found no effect of therapy targeting IL-17 in an unselected population of asthmatic patients but showed a potential benefit in a sub-population of patients exhibiting a high level of airway reversibility, suggesting a potential role on airway remodeling. Anti-IL-22 therapies have not been evaluated in asthma yet but were demonstrated efficient in severe atopic dermatitis including an effect on skin remodeling. In this review, we will address the role of Th17 cytokines in airway remodeling through data from in vitro, in vivo and translational studies, and examine the potential place of Th17-targeting therapies in the treatment of asthma with airway remodeling.
Keywords: Th17 inflammation; airway remodeling; asthma; interleukine-17; interleukine-22.
Copyright © 2022 Margelidon-Cozzolino, Tsicopoulos, Chenivesse and de Nadai.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

