Interstitial lung disease in systemic sclerosis quantification of disease classification and progression with high-resolution computed tomography: An observational study
- PMID: 35386737
- PMCID: PMC8892932
- DOI: 10.1177/2397198320985377
Interstitial lung disease in systemic sclerosis quantification of disease classification and progression with high-resolution computed tomography: An observational study
Abstract
Introduction: Systemic sclerosis-associated interstitial lung disease accounts for up to 20% of mortality in these patients and has a highly variable prognosis. Functional respiratory imaging, a quantitative computed tomography imaging technique which allows mapping of regional information, can provide a detailed view of lung structures. It thereby shows potential to better characterize this disease.
Purpose: To evaluate the use of functional respiratory imaging quantitative computed tomography in systemic sclerosis-associated interstitial lung disease staging, as well as the relationship between short-term changes in pulmonary function tests and functional respiratory imaging quantitative computed tomography with respect to disease severity.
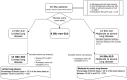
Materials and methods: An observational cohort of 35 patients with systemic sclerosis was retrospectively studied by comparing serial pulmonary function tests and in- and expiratory high-resolution computed tomography over 1.5-year interval. After classification into moderate to severe lung disease and limited lung disease (using a hybrid method integrating quantitative computed tomography and pulmonary function tests), post hoc analysis was performed using mixed-effects models and estimated marginal means in terms of functional respiratory imaging parameters.
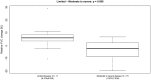
Results: At follow-up, relative mean forced vital capacity percentage change was not significantly different in the limited (6.37%; N = 13; p = 0.053) and moderate to severe disease (-3.54%; N = 16; p = 0.102) groups, respectively. Specific airway resistance decreased from baseline for both groups. (Least square mean changes -25.11% predicted (p = 0.006) and -14.02% predicted (p = 0.001) for limited and moderate to severe diseases.) In contrast to limited disease from baseline, specific airway radius increased in moderate to severe disease by 8.57% predicted (p = 0.011) with decline of lower lobe volumes of 2.97% predicted (p = 0.031).
Conclusion: Functional respiratory imaging is able to differentiate moderate to severe disease versus limited disease and to detect disease progression in systemic sclerosis.
Keywords: Interstitial lung disease; computer-assisted image analysis; multidetector-row computed tomography; systemic scleroderma.
© The Author(s) 2021.
Conflict of interest statement
Authors’ note: This research adds a new perspective on the quantification of disease progression in interstitial lung disease in systemic sclerosis, using a detailed quantitative CT analysis method (functional respiratory imaging). Declaration of conflicting interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: M.L., D.B., C.V.H., and B.R.L. are the employees of FLUIDDA Inc. D.V. reports personal fees and non-financial support from Boehringer Ingelheim, outside the submitted work. J.C., P.C. and W.D.B. have nothing to disclose. J.D.B. is the shareholder and employee of FLUIDDA Inc. D.K. reports personal fees from Actelion, personal fees from Astra Zeneca, grants and personal fees from Bayer, grants and personal fees from BMS, grants and personal fees from Boehringer Ingelheim, personal fees from Chemomab, personal fees from CiVi Biopharma, Inc., personal fees from Corbus, personal fees from CSL Behring, personal fees from Cytori, personal fees from Eicos, personal fees from EMD Serono, grants and personal fees from Genentech/Roche, personal fees from GSK, personal fees from Horizon, grants from NIH NIAID, grants from NIH NIAMS, grants from Pfizer, grants and personal fees from Sanofi-Aventis/Genzyme, personal fees from UCB Pharma, during the conduct of the study, and personal fees from AstraZeneca, outside the submitted work. He is the chief medical officer of Eicos Sciences, Inc.
Figures



References
-
- Denton CP, Khanna D. Systemic sclerosis. Lancet 2017; 390: 1685–1699. - PubMed
-
- Perelas A, Silver RM, Arrossi AV, et al.. Systemic sclerosis-associated interstitial lung disease. Lancet Respir Med 2020; 8: 304–320. - PubMed
-
- Tyndall AJ, Bannert B, Vonk M, et al.. Causes and risk factors for death in systemic sclerosis: a study from the EULAR scleroderma trials and research (EUSTAR) database. Ann Rheum Dis 2010; 69(10): 1809–1815. - PubMed
-
- Bouros D, Wells AU, Nicholson AG, et al.. Histopathologic subsets of fibrosing alveolitis in patients with systemic sclerosis and their relationship to outcome. Am J Respir Crit Care Med 2002; 165: 1581–1586. - PubMed
LinkOut - more resources
Full Text Sources
