Personalized Pollen Monitoring and Symptom Scores: A Feasibility Study in Grass Pollen Allergic Patients
- PMID: 35387060
- PMCID: PMC8974794
- DOI: 10.3389/falgy.2021.628400
Personalized Pollen Monitoring and Symptom Scores: A Feasibility Study in Grass Pollen Allergic Patients
Abstract
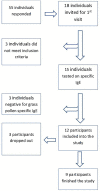
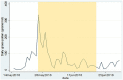
Background: Pollen is a major trigger for allergic symptoms in sensitized individuals. Airborne pollen is usually monitored by Hirst type pollen samplers located at rooftop level, providing a general overview of the pollen distribution in the larger surroundings. In this feasibility study, grass pollen-sensitized subjects monitored the pollen in their direct environment using a portable pollen sampler (Pollensniffer) and scored their symptoms, to study the relation between symptom severity and personal grass pollen exposure. For comparison the symptoms were also correlated with pollen collected by the rooftop sampler. Methods: After recruitment 18 participants were screened for grass pollen specific (GP-sIgE) of which 12 were eligible. Nine participants completed the study (May, 2018). They were asked to monitor personal pollen exposure using a Pollensniffer on their way to school, work or other destination, and to score their symptoms via a mobile app on a scale from 0 to 10. Daily pollen concentrations were collected by a Hirst type sampler at rooftop level. Pollen grains were analyzed using a microscope. Results: Three of the four participants with high GP-sIgE (≥9.6 kU/l) reported high symptom scores (>4) and an analysis showed a significant correlation (CC) between eye, nose, and lung symptoms and the grass pollen counts collected by the Pollensniffer, as well as the daily grass pollen concentrations monitored by the rooftop sampler (CC≥0.54). In contrast, the participants with low GP-sIgE levels (<9.6 kU/l) reported low symptom scores (≤4) and often other sensitizations were present. For these subjects, no significant positive correlations (CC<0.3) of symptoms with either grass pollen collected by the personal or the rooftop sampler were found. Conclusion: The results of this feasibility study suggest that correlations between the severity of clinical symptoms of grass pollen allergic patients, and grass pollen counts as determined by the Pollensniffer or a rooftop sampler, is restricted to patients with high GP-sIgE levels, high symptom scores, and no relevant other sensitizations. Based on the low numbers of subjects with severe symptoms included in this feasibility study, no conclusions can be drawn on the performance of the Pollensniffer in relating symptoms and pollen exposure in comparison with the rooftop sampler. Trial Registration: The study was approved by the Committee Medical Ethics of the LUMC (approval numbers: NL63953.058.17/ P17.304).
Keywords: allergic rhinitis; grass pollen; personal pollen sampler; pollen-induced rhinoconjunctivitis; symptoms scores.
Copyright © 2021 de Weger, van Hal, Bos, Molster, Mostert and Hiemstra.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
LinkOut - more resources
Full Text Sources

