Genetic Landscape of the ACE2 Coronavirus Receptor
- PMID: 35387486
- PMCID: PMC9047645
- DOI: 10.1161/CIRCULATIONAHA.121.057888
Genetic Landscape of the ACE2 Coronavirus Receptor
Erratum in
-
Correction to: Genetic Landscape of the ACE2 Coronavirus Receptor.Circulation. 2022 Jul 5;146(1):e4. doi: 10.1161/CIR.0000000000001081. Epub 2022 Jul 5. Circulation. 2022. PMID: 35858169 Free PMC article. No abstract available.
Abstract
Background: SARS-CoV-2, the causal agent of COVID-19, enters human cells using the ACE2 (angiotensin-converting enzyme 2) protein as a receptor. ACE2 is thus key to the infection and treatment of the coronavirus. ACE2 is highly expressed in the heart and respiratory and gastrointestinal tracts, playing important regulatory roles in the cardiovascular and other biological systems. However, the genetic basis of the ACE2 protein levels is not well understood.
Methods: We have conducted the largest genome-wide association meta-analysis of plasma ACE2 levels in >28 000 individuals of the SCALLOP Consortium (Systematic and Combined Analysis of Olink Proteins). We summarize the cross-sectional epidemiological correlates of circulating ACE2. Using the summary statistics-based high-definition likelihood method, we estimate relevant genetic correlations with cardiometabolic phenotypes, COVID-19, and other human complex traits and diseases. We perform causal inference of soluble ACE2 on vascular disease outcomes and COVID-19 severity using mendelian randomization. We also perform in silico functional analysis by integrating with other types of omics data.
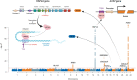
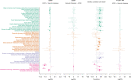
Results: We identified 10 loci, including 8 novel, capturing 30% of the heritability of the protein. We detected that plasma ACE2 was genetically correlated with vascular diseases, severe COVID-19, and a wide range of human complex diseases and medications. An X-chromosome cis-protein quantitative trait loci-based mendelian randomization analysis suggested a causal effect of elevated ACE2 levels on COVID-19 severity (odds ratio, 1.63 [95% CI, 1.10-2.42]; P=0.01), hospitalization (odds ratio, 1.52 [95% CI, 1.05-2.21]; P=0.03), and infection (odds ratio, 1.60 [95% CI, 1.08-2.37]; P=0.02). Tissue- and cell type-specific transcriptomic and epigenomic analysis revealed that the ACE2 regulatory variants were enriched for DNA methylation sites in blood immune cells.
Conclusions: Human plasma ACE2 shares a genetic basis with cardiovascular disease, COVID-19, and other related diseases. The genetic architecture of the ACE2 protein is mapped, providing a useful resource for further biological and clinical studies on this coronavirus receptor.
Keywords: COVID-19; Genome-Wide Association Study; SARS-CoV-2; angiotensin-converting enzyme 2; cardiovascular diseases.
Figures


References
-
- Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH, Nitsche A, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280.e8. doi: 10.1016/j.cell.2020.02.052 - PMC - PubMed
-
- Sungnak W, Huang N, Bécavin C, Berg M, Queen R, Litvinukova M, Talavera-López C, Maatz H, Reichart D, Sampaziotis F, et al. ; HCA Lung Biological Network. SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat Med. 2020;26:681–687. doi: 10.1038/s41591-020-0868-6 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

