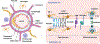Neuroinflammation, Stroke, Blood-Brain Barrier Dysfunction, and Imaging Modalities
- PMID: 35387495
- PMCID: PMC9038693
- DOI: 10.1161/STROKEAHA.122.036946
Neuroinflammation, Stroke, Blood-Brain Barrier Dysfunction, and Imaging Modalities
Abstract
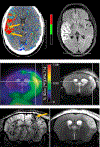
Maintaining blood-brain barrier (BBB) integrity is crucial for the homeostasis of the central nervous system. Structurally comprising the BBB, brain endothelial cells interact with pericytes, astrocytes, neurons, microglia, and perivascular macrophages in the neurovascular unit. Brain ischemia unleashes a profound neuroinflammatory response to remove the damaged tissue and prepare the brain for repair. However, the intense neuroinflammation occurring during the acute phase of stroke is associated with BBB breakdown, neuronal injury, and worse neurological outcomes. Here, we critically discuss the role of neuroinflammation in ischemic stroke pathology, focusing on the BBB and the interactions between central nervous system and peripheral immune responses. We highlight inflammation-driven injury mechanisms in stroke, including oxidative stress, increased MMP (matrix metalloproteinase) production, microglial activation, and infiltration of peripheral immune cells into the ischemic tissue. We provide an updated overview of imaging techniques for in vivo detection of BBB permeability, leukocyte infiltration, microglial activation, and upregulation of cell adhesion molecules following ischemic brain injury. We discuss the possibility of clinical implementation of imaging modalities to assess stroke-associated neuroinflammation with the potential to provide image-guided diagnosis and treatment. We summarize the results from several clinical studies evaluating the efficacy of anti-inflammatory interventions in stroke. Although convincing preclinical evidence suggests that neuroinflammation is a promising target for ischemic stroke, thus far, translating these results into the clinical setting has proved difficult. Due to the dual role of inflammation in the progression of ischemic damage, more research is needed to mechanistically understand when the neuroinflammatory response begins the transition from injury to repair. This could have important implications for ischemic stroke treatment by informing time- and context-specific therapeutic interventions.
Keywords: blood-brain barrier; immunity; ischemic stroke; microglia; neuroinflammatory diseases.
Conflict of interest statement
Disclosures
The authors report no conflicts.
Figures