Development of a Bead-Based Multiplex Assay for Use in Multianalyte Screening and Surveillance of HIV, Viral Hepatitis, Syphilis, and Herpes
- PMID: 35387497
- PMCID: PMC9116187
- DOI: 10.1128/jcm.02348-21
Development of a Bead-Based Multiplex Assay for Use in Multianalyte Screening and Surveillance of HIV, Viral Hepatitis, Syphilis, and Herpes
Abstract
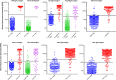
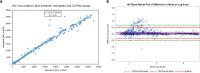
Diagnostic assays that can simultaneously determine the presence of infection with multiple pathogens are key for diagnosis and surveillance. Current multiplex diagnostic assays are complex and often have limited availability. We developed a simple, multianalyte, pathogen detection assay for screening and serosurveillance using the Luminex Magpix platform that is high throughput and can be helpful in monitoring multiple diseases. The Luminex bead-based 10-plex immunoassay for the detection of HIV-1, HIV-2, Treponema pallidum, hepatitis B virus (HBV), hepatitis C virus (HCV), herpes simplex virus 1 (HSV-1), and HSV-2 infections was accomplished by coupling beads with specific antigens to detect IgG antibodies in plasma or serum samples. Each coupled antigen was systematically optimized, and the performance was evaluated using a panel of well-characterized specimens (n = 417) that contained antibodies to HIV-1, HIV-2, T. pallidum, HBV, HCV, HSV-1, and HSV-2. The multiplex assay had a sensitivity of 92.2% (95% Clopper-Pearson confidence interval [CI], 90.2 to 94.0%) and a specificity of 98.1% (95% CI, 97.6 to 98.7%). The sensitivities and specificities for disease-specific biomarker detection ranged from 68.7 to 100% and 95.6 to 100%, respectively. The results showed that the 10-plex immunoassay had an overall agreement of 96.7% (95% CI, 96.7 to 97.3%) with reference tests and a corresponding kappa value of 0.91 (95% CI, 0.90 to 0.93). Kappa values for the individual pathogens ranged from 0.69 to 1.00. The assay is robust and allows the simultaneous detection of antibodies to multiple antigens using a small sample volume in a high-throughput format. This assay has the potential to simplify disease surveillance by providing an alternative to expensive and highly specialized individual tests.
Keywords: blood-borne; multipathogen; multiplex.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Woodring J, Ishikawa N, Nagai M, Malarski M, Takashima Y, Sobel H, Lo Y-R. 2017. Integrating HIV, hepatitis B and syphilis screening and treatment through the Maternal, Newborn and Child Health platform to reach global elimination targets. Western Pac Surveill Response J 8:1–5. 10.5365/wpsar.2017.8.3.005. - DOI - PMC - PubMed
-
- WHO. 2017. Regional framework for the triple elimination of mother-to-child transmission of HIV, hepatitis B and syphilis in Asia and the Pacific 2018-2030. WHO, Geneva, Switzerland.
-
- WHO. 2016. Global health sector strategy on viral hepatitis 2016-2021. WHO, Geneva, Switzerland.
-
- UNAIDS. 2017. AIDS info 2015. UNAIDS, Geneva, Switzerland. http://www.aidsinfo.unaids.org.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

