Enhancement of prime editing via xrRNA motif-joined pegRNA
- PMID: 35387980
- PMCID: PMC8986804
- DOI: 10.1038/s41467-022-29507-x
Enhancement of prime editing via xrRNA motif-joined pegRNA
Abstract
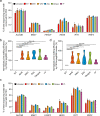
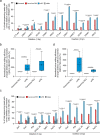
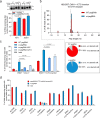
The prime editors (PEs) have shown great promise for precise genome modification. However, their suboptimal efficiencies present a significant technical challenge. Here, by appending a viral exoribonuclease-resistant RNA motif (xrRNA) to the 3'-extended portion of pegRNAs for their increased resistance against degradation, we develop an upgraded PE platform (xrPE) with substantially enhanced editing efficiencies in multiple cell lines. A pan-target average enhancement of up to 3.1-, 4.5- and 2.5-fold in given cell types is observed for base conversions, small deletions, and small insertions, respectively. Additionally, xrPE exhibits comparable edit:indel ratios and similarly minimal off-target editing as the canonical PE3. Of note, parallel comparison of xrPE to the most recently developed epegRNA-based PE system shows their largely equivalent editing performances. Our study establishes a highly adaptable platform of improved PE that shall have broad implications.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

